Abstract
Objective
The aim of the study was to evaluate the effects of vitamin D deficiency on the mandibular bone structure by fractal analysis and panoramic morphometric indices.
Methods
Ninety participants were divided into three groups as 30 individuals with severe vitamin D deficiency, 30 individuals with vitamin D deficiency, and 30 individuals with vitamin D sufficiency. Fractal dimension analysis (FD), panoramic mandibular index (PMI), mandibular cortical index (MCI), and mandibular cortical thickness measurement (CTM) were evaluated on panoramic radiographs.
Results
FD values of the patients with vitamin D deficiency were found to be statistically lower than the patients with vitamin D sufficiency (p < 0.05). FD value of supracortical area above the angulus mandible (FD2) in patients with severe vitamin D deficiency was significantly lower than FD values (p = 0.002). There was no statistically significant difference between the groups in the CTM (p > 0.05). PMI was significantly lower in patients with severe vitamin D deficiency (p < 0.001). There was a significant difference in MCI values between the groups (p < 0.05).
Conclusion
Vitamin D deficiency causes a decrease in bone mineral density in the mandible, and an increase in alveolar porosity. FD analysis and radiomorphometric indices in panoramic radiographs can be used to assess osteoporotic changes in patients with vitamin D deficiency.
Similar content being viewed by others
Introduction
Vitamin D is a steroid hormone and its deficiency is a global public health problem. For many years, Vitamin D has been known for its important role in regulating calcium and phosphorus levels in the body and bone mineralization. There are two forms of vitamin D: Vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). Vitamin D3 is mostly synthesized in the skin via ultraviolet irradiation of 7-dehydrocholesterol. Both forms can be absorbed from the diet and enter the bloodstream [1]. Vitamin D is metabolized in the liver and converted into 25-hydroxyvitamin D (25(OH)D), which is the best indicator of the amount of vitamin D stores in the body. Then, 25(OH)D is converted to 1.25(OH)2D3 which is the biologically active form in the kidneys. Vitamin D plays role in various physiological processes including cellular growth and differentiation, cardiovascular function, immunity, and calcium metabolism [2].
25(OH)D is the major circulating form of vitamin D and its main sources are sun exposure and vitamin D intake by diet. Therefore, it is used to determine the vitamin D level in the body [3]. The prevalence of vitamin D deficiency is increasing globally. It is estimated that approximately 1 billion people have vitamin D deficiency or insufficiency [4, 5].
Vitamin D, as an important component of the interactions between kidney, bone, parathyroid gland, and intestine, helps regulate the skeletal and blood mineral balance. It is also required for the maintenance of skeletal integrity through its role in regulating extracellular calcium levels. Vitamin D is essential for the development and maintenance of the skeletal system since it helps create the optimal conditions for bone mineralization by increasing the absorption of calcium and phosphate in the small intestine [6]. While calcium absorption is around 10–15% in the absence of vitamin D, it increases to 30–80% in the presence of adequate vitamin D [7].
A low level of 25(OH)D causes conditions such as increased parathyroid hormone (PTH) secretion, increased bone resorption, osteoporosis, osteomalacia, increased fractures in the hip or other bones [8, 9].
Fractal dimension (FD) analysis method assesses the trabecular bone tissue with the box-counting algorithm and clarifies the complexity of fractal structures including bone. Higher FD values have been associated with a more complex bone structure [10]. The FD analysis method has been shown to be capable in the determination of osteoporotic conditions in the jaws [11]. For this purpose, panoramic radiographs are generally used [12]. Mandibular cortical index (MCI) describes the severity of cortical erosion. It can be a practical indicator for detecting osteoporotic conditions, providing information about the quality and quantity of bone. The panoramic mandibular index (PMI) has been described as a useful method for estimating bone mineral density [13]. Fractal analysis and mandibular indexes can be measured on existing panoramic images of patients, therefore extra imaging is not required [11,12,13].
Evaluating osteoporotic changes in the jaw bone before creating a dental treatment plan in patients with vitamin D deficiency is important because dental therapies such as periodontal treatment and implant surgery are affected by bone quality [14,15,16].
According to our knowledge, there is no study in the literature evaluating the effect of vitamin D deficiency on jawbones using FD analysis. The aim of this study is to evaluate the mandibular bone structure of patients with different severities of vitamin D deficiency by FD analysis and panoramic morphometric indices such as mandibular cortical thickness measurement (CTM), PMI, MCI, compared with the systemically healthy control group.
Materials and methods
Study group selection
This retrospective study was conducted in Recep Tayyip Erdogan University Faculty of Dentistry, Department of Oral and Maxillofacial Radiology in full accordance with the applicable ethical principles, including the World Medical Association Declaration of Helsinki of 1964 and later versions. The study protocol was approved by Research Ethics Committee of the Recep Tayyip Erdogan University. (decision no:2021/107) The data were obtained from the archives of patients who previously applied to the department of Oral and Maxillofacial Radiology for various reasons such as toothache, periodontal treatment, implant surgery, etc. Information about serum 25(OH)D levels in blood was obtained from medical history records. Ninety participants were included in the study. They were divided into 3 groups for 30 patients per each: Vitamin D values less than 10 ng/ml were accepted as severe deficiency (Group 1); between 10 and 20 ng/ml as deficiency (Group 2); and values of 30 ng/ml and above were accepted as sufficient values [14]. For each group, 15 female and 15 male patients aged between 18–45 were selected. Panoramic radiographs with sufficient diagnostic quality, in which foramen mentale could be clearly observed and all cortical structures could be evaluated were included in the study.
Only images of patients without intraosseous pathology in the areas where the fractal analysis would be performed were included. Patients with a history of a systemic disease that could affect the bone structure or usage of a medication that could affect bone mineralization were excluded. Metabolic diseases that may affect bone density such as diabetes, osteoporosis, or hypo/hyperparathyroidism; temporomandibular joint diseases that may affect the subcondylar region; mandibular pathologies such as cyst and tumor; those with less than 20 teeth, or those with inadequate diagnostic quality panoramic radiographs were also excluded from the study.
All panoramic radiographs were obtained with the same device (Planmeca Promax 2D S2, Planmeca Oy; Helsinki, Finland) by applying the same exposure parameters (66 kVp, 8 mA, 16.6 s), according to the manufacturer's instructions and by adjusting the Frankfurt horizontal plane parallel to the ground and the vertical line to the sagittal plane.
Power analysis was performed with a software (GPower 3.1.0, Universitat Dusseldorf, Germany) to determine the number of individuals included in the study. Sample size calculation was based on the ability to detect significant differences at α = 0.05 error probability. According to the power analysis, a sample size of 90 patients would give more than 93% power (actual power: 0.9264).
Image evaluation
Fractal dimension analysis
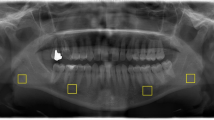
In this method, images were analyzed by a software (ImageJ v1.52, National Institutes of Health, Bethesda, United States). The program was downloaded from the following web address: https://imagej.nih.gov/ij/download.html. Measurements were made on 4 different regions of interest (ROI) areas from the right and left sides of the panoramic radiographs of each patient. The mean value of both measurements represented the average of the FD. Respectively as ROI; 45 × 45 pixel areas were selected on the trabecular bone within the subcortical area in the condyle (FD1), the supracortical area above the angulus mandible (FD2), above the mandibular canal distal side to the second premolar (FD3), anterior to the mental foramen (FD4). The box-counting method was preferred for fractal analysis on each ROI area as suggested by White and Rudolph [17]. Both sets of panoramic radiographs were converted to tagged image file formats due to their high resolution. Each ROI was selected, cropped, and duplicated. Gaussian Blur was performed to remove the brightness changes depending on the upper soft tissues and varying bone thicknesses. The resulting image was then removed from the original image. Bone marrow cavities and trabeculae were separated from each other by adding 128 Gy values to each pixel location. FD analysis was calculated after performing binary, erode, dilate, invert, and skeletonize operations. The image was divided into pixel squares and the frames containing trabeculae and the total number of frames were calculated for each different sized pixel. The slope of the line graphed in logarithmic scale according to the values obtained gave the FD value (Fig. 1).
A Selected regions for fractal analysis in panoramic radiography: subcortical area in the condyle, subcortical area of the angulus mandible, posterior and anterior mental foramen B Image j program used for fractal analysis C From left to right for the top row; Addition Gaussian blur filter, Addition of a gray value of 128 to each pixel location, Binarization. From left to right for the top row; Erosion, Inversion, Skeletonizion. D Fractal analysis with box-counting method
Radiomorphometric analysis
The morphometric measurement has been carried out using Planmeca Romexis 4.6.2.R software (PLANMECA Romexis, Helsinki, Finland). The cortical thickness measurements were carried out on panoramic radiography as performed on cone-beam computed tomography (CBCT) by Barra et al. [18]. Accordingly, radiomorphometric indices were measured on symphysis, anterior, molar, and posterior regions of the in right and left side of jaws and the mean of both measurements was represented as the mean value. The symphysis index (SI) was measured perpendicular to the inferior mandibular cortex, equidistant from the right and left mental foramen centers. Anterior index (AI) was measured perpendicular to the inferior cortex of the mandible, 1 cm in front of the mental foramen, parallel to the long axis of the mandible. The molar index (MI) was measured 1 cm behind the mental foramen, parallel to the long axis of the mandible, and perpendicular to the inferior cortex of the mandible. And the posterior index (PI) was measured 2.5 cm behind the mental foramen, parallel to the long axis of the mandible, and perpendicular to the inferior cortex of the mandible (Fig. 2) [18].
The PMI is the ratio of the distance between the mandibular cortical width (MCW; it is a line perpendicular to the inferior border of the mandible at the center of the mental foramen) by the distance between the inferior border of the mental foramen and the inferior mandibular cortex (Fig. 2). The reason for choosing the mental foramen area is that there are no masticatory muscles attached to this area [19]. The average of two different measurements for the right and the left side represents the mean value of the PMI.
The MCI also known as Klemetti index consists of 3 subgroups classified by Klemetti et al. According to this classification; C1 = normal cortex: the endosteal margin of the cortex is matched and tapered on both sides; C2 = moderately worn cortex: endosteal margin, lacunar resorption resulting in semilunar defects or formation of endosteal cortical residues; C3 = over eroded, eroded or porous cortex: forms dense layers of the cortex, endosteal, and clearly porous cortical remnants (Fig. 3) [13].
All measurements were made by an experienced oral and maxillofacial radiologist (DNG) who was blind to information about patients. All analyses were performed at the same time and to assess intra-observer agreement, randomly selected 20% of panoramic images were reevaluated 2 weeks later.
Statistical analysis
The statistical analyses were conducted with SPSS for Windows SPSS 8 version 23.0 (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL) with a level of significance of 5% (p < 0.05). For evaluation of intra-observer agreement Cohen’s kappa was used for MCI, intraclass correlation coefficient (ICC) was used for FD, CTM, PMI. One-Way ANOVA test was used to compare all measurements between the three groups. Then, the Duncan test was conducted to find out which groups caused the difference. Repeated measures ANOVA was used to evaluate FD values and CTM within groups. Benferroni Post Hoc test was used to determine the values that make the difference. Chi-square analysis was applied to determine the relationship between categorical variables.
Results
The study included a total of 90 individuals. The mean age ± standard deviations of the individuals whose 25(OH)D values are less than 10 ng/ml, between 10-20 ng/ml, 30 ng/ml and above were respectively; 28.8 ± 8.7, 32.6 ± 9.9, 33.1 ± 9.1. There was no difference between the groups in age and gender. After re-evaluation for intra-observer reliability, the weighted Kappa coefficients were found to be 0.878 for MCI. ICC was 0, 87, 0.868, and 0.874 for FD analysis, CTM and PMI respectively and they were good intra-observer reliability [20].
There was a statistically significant difference in FD values between the groups (p < 0.05). While the lowest FD values were seen in group 1, the highest FD values were seen in group 3 (Table 1). FD2 value was significantly lower than other measurement sites in group 1 (p = 0.002). No statistical difference was found in the measurements of the other three regions (Table 1).
There was no difference between cortical thickness measurements of the groups (p > 0.05). In all three groups, the posterior index (PI) value had the lowest value compared to other measurements, and there was a statistically significant difference (p < 0.001). A statistically significant difference was observed in PMI between the groups (p < 0.001). PMI value in group 3 was higher than group 1 and group 2 and there was a statistically significant difference (p < 0.001) (Table 1).
The results of MCI to groups was presented in Table 2. There was a significant difference in C1 and C2 index values between the groups (p < 0.001). While the number of individuals with C1 index was found dominant in group 3 (59.6%), it was least in group 1 (14.9%). The number of individuals with C2 index was dominant in group 1 (53.5%), whereas it was least in group 3 (4.7%). While C2 was seen in most of the people in group 1 (76.7%), most of the people in group 3 had C1 (93.3%). C1 and C2 were similar in group 2 (p > 0.05).
Discussion
Vitamin D deficiency is known to affect many people and diseases. Even in the biggest pandemic of recent years, Vitamin D deficiency is thought to be associated with COVID-19 disease because of its effects on both innate and adaptive immunity and studies have been conducted on this issue [21, 22]. Another important role of vitamin D is maintaining bone strength and mineral balance. Vitamin D deficiency can cause rickets, osteomalacia, and an increased incidence of bone fracture. If calcium absorption from the intestine is insufficient, PTH and 1,25(OH)2D3 can stimulate osteoclastogenesis. Thus, there is a transition of calcium from bone to blood [1, 8, 9].
In this type of studies examiner reproducibility is needed for the results to be more reliable and interpretable. Therefore, all the measurements performed by the same radiologist at different times and high intra-observer agreement was observed.
There are several studies about the methods for analyzing the bone in terms of qualitative and quantitative parameters in dentistry [23,24,25,26,27,28,29,30,31,32,33]. In a study by Alman et al. [30] it was reported that FD analysis performed in the mandibular regions has a good diagnostic ability to detect patients with low bone mineral density. Also in another study, Magat et al.[34] evaluated FD analysis using both CBCT and panoramic radiography. They suggested that since CBCT has higher radiation dose and lower image resolution, panoramic radiography is more advantageous for the examination of trabecular bone. In the literature, there are also many studies that panoramic morphometric indices were used successfully to deduce possible disease or drug-related osteoporotic conditions in craniofacial bones [31, 35].
According to our knowledge, this study is the first to quantify radiomorphometric indices and analyze FD on panoramic radiographs in patients with vitamin D deficiency.
Demiralp et al.[33] reported that FD values were higher in patients using bisphosphonates. This may be due to decreased bone resorption in people using bisphosphonates. In some studies FD values were found to be lower in patients with chronic renal failure [32], thalassemia major [36], sickle cell anemia [37] compared to patients in the control group. According to the study of Ustaoglu et al., lower FD values were reported in the regions of subcortical area in condyle, supracortical area above angulus, and above the mandibular canal distal side to the second premolar in patients with antiepileptic drug-induced osteoporosis [12]. The study reported by Göller et al. found that mean FD value of mandible and value of supracortical area above angulus and anterior mental foramen was significantly lower in patients using aromatase inhibitors [38]. In a study conducted by Coşgunarslan et al., the FD values of ramus and angulus mandible were significantly lower in patients using selective serotonin reuptake inhibitors (SSRIs). There was no significant difference between the study and control groups for the mean FD value of mandibular cortical bone [10]. Contraversely in another study conducted on women with celiac disease by Neves et al. [39], no statistically significant difference was found in FD value between the study and control groups. In the present study, the mean FD value and and FD2 value were lower in the groups with vitamin D deficiency and it was statistically significant (p < 0.05). This supports the view that vitamin D deficiency causes a decrease in bone mineral density and increases alveolar porosity.
In the literature, there are many studies investigating bone mineral density with panoramic radiography by MCI, MCW, PMI [13, 19, 31,32,33, 40,41,42]. According to the results of the studies, the MCW value was found to be lower in patients with osteoporosis [17, 32, 41, 43, 44]. In our study, we used the new radiomorphometric indices used by Barra et al.[18] instead of MCW. According to the results of our study, there was no difference in CTM values between the groups (p > 0.05). Barra et al.[18] reported that the MI and PI indices in CBCT can be used to determining low bone mineral density in postmenopausal women.
In the literature there are studies that found no difference in PMI in the control and study groups [10, 11, 36, 39], as well as studies that found that the PMI was higher in the control group compared to the study group [12, 38]. In the present study the PMI value in Group 1 was higher than the others.and there was a statistically significant difference between the groups (p < 0.05).
In our study, there was a significant difference in MCI between the groups. (p < 0.05). Similarly, Cakur et al. reported that MCI is significantly related to vertebral bone mineral density in osteoporotic women [45]. Also in another study among postmenapausal women with osteoporosis, Klemetti et al. found that MCI is strongly associated with buccal cortex bone mineral density [24]. Contraversely in some studies that evaluates MCI, there was no difference between the groups [11, 12, 36, 38].
Vitamin D levels have been investigated in some studies regarding the existence of its association with periodontal disease and their results showed that serum levels of vitamin D were significantly lower in patients with periodontitis than those in healthy subjects [46, 47]. Gong et al. concluded that 1,25-Dihydroxyvitamin D deficiency accelerated alveolar bone loss by inhibiting osteoblastic bone formation and enhancing periodontal tissue degeneration in calcium and phosphorus as well as age-independent manner [48]. In addition, poor bone mineralization may be a factor that facilitate the destruction in periodontal disease. Furthermore, Mangano et al. [15] researched correlation between early implant failure and low serum levels of vitamin D and showed a higher incidence of the implant failure rate. Osteoporotic changes occurred by vitamin D deficiency may be one of the reasons for failure in dental treatment such as implant surgery or periodontal therapy. Vitamin D supplementation may positively affect dental treatment results in those with vitamin D deficiency.
There are some limitations of this study. First one is that since it is a retrospective investigation there were not enough patients for insufficient group with the Vitamin D values between 21 and 29 ng/ml. Therefore, the insufficient group was ommitted from the study. Another limitation is that we studied on only the mandible, new comprehensive investigations can be conducted including maxilla to have more information about the effects of vitamin D deficiency on jaws.
In conclusion; according to results of radiologic examinatinations in present study, Vitamin D deficiency effects the trabeculation, porosity, mineral bone density of mandible. Fractal analyze and the other morphometric indices can be used for evaluating the alveolar structure in panoramic radiographs. To have more detailed information about the effects of vitamin D deficiency on alveolar bone further studies is needed for mandible and maxilla with larger populations.
References
Goltzman D. Functions of vitamin D in bone. Histochem Cell Biol. 2018;149(4):305–12.
Amano Y, Komiyama K, Makishima M. Vitamin D and periodontal disease. J Oral Sci. 2009;51(1):11–20.
Holick MF. Vitamin D status: Measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73–8.
Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81.
Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J Clin Endocrinol Metab. 2012;97(4):1153–8.
Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289(1):8–28.
Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69(5):842–56.
Boucher BJ. Inadequate vitamin D status: does it contribute to the disorders comprising syndrome “X”? Br J Nutr. 1998;79(4):315–27.
McGrath J, Feron F, Eyles D, Mackay-Sim A. Vitamin D: the neglected neurosteroid? Trends Neurosci. 2001;24(10):570–2.
Coşgunarslan A, Aşantoğrol F, Soydan Çabuk D, Canger EM. The effect of selective serotonin reuptake inhibitors on the human mandible. Oral Radiol. 2021;37(1):20–8.
Coşgunarslan A, Canger EM, Soydan Çabuk D, Kış HC. The evaluation of the mandibular bone structure changes related to lactation with fractal analysis. Oral Radiol. 2020;36(3):238–47.
Ustaoğlu G, Göller Bulut D, Bayrak S. Applicability of dental panoramic radiomorphometric and fractal dimension analysis in the evaluation of antiepileptic drug-induced osteoporosis. Int J Med Dent. 2019;23(2):201–7.
Klemetti E, Kolmakow S. Morphology of the mandibular cortex on panoramic radiographs as an indicator of bone quality. Dentomaxillofac Radiol. 1997;26(1):22–5.
Anand N, Chandrasekaran SC, Rajput NS. Vitamin D and periodontal health: Current concepts. J Indian Soc Periodontol. 2013;17(3):302–8.
Mangano F, Mortellaro C, Mangano N, Mangano C. Is low serum vitamin d associated with early dental implant failure? a retrospective evaluation on 1625 implants placed in 822 patients. Mediators Inflamm. 2016;2016:5319718.
Wagner F, Schuder K, Hof M, Heuberer S, Seemann R, Dvorak G. Does osteoporosis influence the marginal peri-implant bone level in female patients? A cross-sectional study in a matched collective. Clin Implant Dent Relat Res. 2017;19(4):616–23.
White SC, Rudolph DJ. Alterations of the trabecular pattern of the jaws in patients with osteoporosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88(5):628–35.
Barra SG, Gomes IP, Amaral TMP, Brasileiro CB, Abreu LG, Mesquita RA. New mandibular indices in cone beam computed tomography to identify low bone mineral density in postmenopausal women. Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;131(3):347–55.
Benson BW, Prihoda TJ, Glass BJ. Variations in adult cortical bone mass as measured by a panoramic mandibular index. Oral Surg Oral Med Oral Pathol. 1991;71(3):349–56.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74.
Bilezikian JP, Bikle D, Hewison M, et al. Mechanisms in endocrinology: Vitamin D and COVID-19. Eur J Endocrinol. 2020;183(5):R133-r147.
Lau FH, Majumder R, Torabi R, et al. Vitamin D insufficiency is prevalent in severe COVID-19. MedRxiv. 2020. https://doi.org/10.1101/2020.04.24.
Çağlayan F, Dağistan S, Keleş M. The osseous and dental changes of patients with chronic renal failure by CBCT. Dentomaxillofac Radiol. 2015;44(5):20140398.
Klemetti E, Kolmakov S, Kröger H. Pantomography in assessment of the osteoporosis risk group. Scand J Dent Res. 1994;102(1):68–72.
White SC. Oral radiographic predictors of osteoporosis. Dentomaxillofac Radiol. 2002;31(2):84–92.
Zeytinoğlu M, İlhan B, Dündar N, Boyacioğlu H. Fractal analysis for the assessment of trabecular peri-implant alveolar bone using panoramic radiographs. Clin Oral Investig. 2015;19(2):519–24.
Soylu E, Coşgunarslan A, Çelebi S, Soydan D, Demirbaş AE, Demir O. Fractal analysis as a useful predictor for determining osseointegration of dental implant? A retrospective study. Int J Implant Dentist. 2021;7(1):14.
Toghyani S, Nasseh I, Aoun G, Noujeim M. Effect of image resolution and compression on fractal analysis of the periapical bone. Acta İnform Med. 2019;27(3):167–70.
Kış HC, Güleryüz GA. Evaluation of the peri-implant bone trabecular microstructure changes in short implants with fractal analysis. Int J Implant Dentist. 2020;6(1):13.
Alman AC, Johnson LR, Calverley DC, Grunwald GK, Lezotte DC, Hokanson JE. Diagnostic capabilities of fractal dimension and mandibular cortical width to identify men and women with decreased bone mineral density. Osteoporos Int. 2012;23(5):1631–6.
Apolinário AC, Sindeaux R, de Souza Figueiredo PT, et al. Dental panoramic indices and fractal dimension measurements in osteogenesis imperfecta children under pamidronate treatment. Dentomaxillofac Radiol. 2016;45(4):20150400.
Gumussoy I, Miloglu O, Cankaya E, Bayrakdar IS. Fractal properties of the trabecular pattern of the mandible in chronic renal failure. Dentomaxillofac Radiol. 2016;45(5):20150389.
Demiralp K, Kurşun-Çakmak E, Bayrak S, Akbulut N, Atakan C, Orhan K. Trabecular structure designation using fractal analysis technique on panoramic radiographs of patients with bisphosphonate intake: a preliminary study. Oral Radiol. 2019;35(1):23–8.
Magat G, Ozcan SS. Evaluation of trabecular pattern of mandible using fractal dimension, bone area fraction, and gray scale value: comparison of cone-beam computed tomography and panoramic radiography. Oral Radiol. 2019;35(1):35–42.
Gupta B, Acharya A, Singh S, et al. Evaluation of jawbone morphology and bone density indices in panoramic radiographs of selective serotonin reuptake inhibitor users: a preliminary study. Dentomaxillofac Radiol. 2019;48(1):20170360.
Bayrak S, Göller Bulut D, Orhan K, et al. Evaluation of osseous changes in dental panoramic radiography of thalassemia patients using mandibular indexes and fractal size analysis. Oral Radiol. 2020;36(1):18–24.
Demirbaş AK, Ergün S, Güneri P, Aktener BO, Boyacioğlu H. Mandibular bone changes in sickle cell anemia: fractal analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(1):e41-48.
Göller Bulut D, Bayrak S, Uyeturk U, Ankarali H. Mandibular indexes and fractal properties on the panoramic radiographs of the patients using aromatase inhibitors. Br J Radiol. 2018;91(1091):20180442.
Neves FS, Barros AS, Cerqueira GA, et al. Assessment of fractal dimension and panoramic radiomorphometric indices in women with celiac disease. Oral Radiol. 2020;36(2):141–7.
Nair VV, Thomas S, Thomas J, Mathew CM. Panoramic radiographs for detecting osteopenia: A pilot study. Clin Pract. 2017;7(4):973.
Ferreira Leite A, de Souza Figueiredo PT, Ramos Barra F, Santos de Melo N, de Paula AP. Relationships between mandibular cortical indexes, bone mineral density, and osteoporotic fractures in Brazilian men over 60 years old. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(5):648–656.
Carmo JZB, Medeiros SF. Mandibular inferior cortex erosion on dental panoramic radiograph as a sign of low bone mineral density in postmenopausal women. Rev Bras Ginecol Obstet. 2017;39(12):663–9.
Taguchi A, Suei Y, Ohtsuka M, Otani K, Tanimoto K, Ohtaki M. Usefulness of panoramic radiography in the diagnosis of postmenopausal osteoporosis in women. Width and morphology of inferior cortex of the mandible. Dentomaxillofac Radiol. 1996;25(5):263–7.
Alonso MB, Cortes AR, Camargo AJ, Arita ES, Haiter-Neto F, Watanabe PC. Assessment of panoramic radiomorphometric indices of the mandible in a brazilian population. ISRN Rheumatol. 2011; 854287.
Cakur B, Sahin A, Dagistan S, et al. Dental panoramic radiography in the diagnosis of osteoporosis. Int Med Res. 2008;36(4):792–9.
Isola G, Alibrandi A, Rapisarda E, Matarese G, Williams RC, Leonardi R. Association of vitamin D in patients with periodontitis: A cross-sectional study. J Periodontal Res. 2020;55(5):602–12.
Boggess KA, Espinola JA, Moss K, Beck J, Offenbacher S, Camargo CA Jr. Vitamin D status and periodontal disease among pregnant women. J Periodontol. 2011;82(2):195–200.
Gong A, Chen J, Wu J, et al. 1,25-dihydroxyvitamin D deficiency accelerates alveolar bone loss independent of aging and extracellular calcium and phosphorus. J Periodontol. 2018;89(8):983–9.
Acknowledgements
Not applicable
Funding
The study was funded solely by the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflict of interest.
Ethics approval
This study was approved by Research Ethics Committee of the Recep Tayyip Erdogan University. (Decision no: 2021/107). All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Informed consent
All participants signed the informed consent form.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zihni Korkmaz, M., Yemenoğlu, H., Günaçar, D.N. et al. The effects of vitamin D deficiency on mandibular bone structure: a retrospective radiological study. Oral Radiol 39, 67–74 (2023). https://doi.org/10.1007/s11282-022-00602-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11282-022-00602-5