Abstract
There is growing recognition that elevated dissolved sulphate around mines will lead to significant environmental issues within the industry that need specific management. Various sulphate attenuation engineering methods exist, and so processes that could occur within a mine site are of particular interest. In this pilot study, we investigate the possibility of using high-sulphate mine water (~ 2500 mg/L) for irrigation of farm land. This approach is made feasible from mildly alkaline (pH ~ 8), non-toxic discharge waters at a semi-arid gold mine site. Geochemical models predicted Ca-sulphate will readily precipitate from the mine water at pH 6 and potentially attenuate downstream concentrations of dissolved sulphate. We used the slightly acidic natural soil environment (pH 5–6) of the mine to test initial model predictions in the laboratory and field trials. Ca-sulphate in gypsum formed in experimental soil columns, and the pH of input water lowered by 2–3 units. In the field, only minor soil gypsum was observed, and instead Mg-sulphate and Ca-carbonate were found more readily on evaporation surfaces. Additional terrestrial effects of soil dilution, leaching, and plant uptake of sulphate are discussed. Irrigated pasture had healthy, green plants that supported a potentially dual benefit of mine water irrigation to enhancing farm productivity in the local community. Irrigation is an effective way to manage mine waters with high dissolved sulphate but requires further trials to refine methods and the feed values of pasture.
Similar content being viewed by others
1 Introduction
Elevated dissolved sulphate in mine waters is widespread around mines that contain sulphides, and there is growing recognition that this is a potential environmental issue (Bowell, 2004; Canovas et al., 2010; Fernando et al., 2018; Jerz & Rimstidt, 2003; Smith et al., 2013; Tolonen et al., 2015, 2016). High dissolved sulphate is most apparent at mine sites with acid mine drainage (Dold, 2017; Hammarstrom et al., 2005; Lottermoser, 2010; Parbhakar-Fox & Lottermoser, 2015; Prasad et al., 2021). Abundant carbonate minerals at some mine sites can neutralise acid, but the issue of elevated sulphate still persists (Elghali et al., 2018; Fernando et al., 2018; Jamieson et al., 2015; Parbhakar-Fox & Lottermoser, 2015). Extraction of dissolved sulphate before water is discharged can be a difficult problem, for which numerous engineering methods have been devised with varying degrees of technical and economic success (Bowell, 2004; Fernando et al., 2018; Tolonen et al., 2015, 2016).
Dissolved sulphate concentrations in acid waters can be limited to some extent by precipitation of sulphate minerals, and this can be enhanced by evaporation (Canovas et al., 2010; Hammarstrom et al., 2005; Hudson-Edwards et al., 1999; Jerz & Rimstidt, 2003). However, waters with circumneutral to mildly alkaline pH commonly contain abundant dissolved carbonate as well as sulphate, and carbonate mineral precipitation can therefore inhibit precipitation of sulphate minerals (e.g. Weightman et al., 2020). This issue can be overcome by artificially raising pH even higher, although this has significant costs and technical issues (Tolonen et al., 2015, 2016).
In this study, we address the issue of elevated dissolved sulphate management of circumneutral to mildly alkaline mine waters at a mine site with a dry climate and high potential for formation of evaporative minerals (Fig. 1a–d). In a previous study (Weightman et al., 2020), we have characterised the geochemical evolution of the waters and their evaporation mineralogy. From that study, the issue of precipitation of carbonate minerals, rather than sulphate minerals, became abundantly clear, and we proposed engineered acidification of water pH as a potential solution, from geochemical models. In this study, we investigate the possibility of using the high-sulphate mine water in a soil environment with a general pH range 2–3 units lower than the water (around pH 5–6) (Fig. 1e), in order to enhance removal of sulphate by evaporative mineral precipitation. His approach potentially has a dual benefit as the surrounding farms have their productivity inhibited by limited water supply and could benefit from an irrigation regime. This paper reports on a pilot irrigation programme to gain indications of the likely water application rates that are practical for both pasture growth and sulphate sequestration. Future follow-up research using the results of this study will investigate the irrigation programme from the perspective of animal health and pasture production.
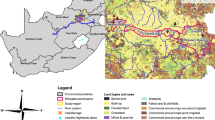
General setting for this study. (a) Location of the Macraes gold mine site in southern New Zealand. (b) Map of the mine site with principal water and irrigation localities referred to in the text. (c) Rainfall map of southern South Island (from NIWA, 2012) showing the rain shadow to the east of mountains. (d) Oblique view from southeast of topography of southern South Island, showing solar irradiation variations (from Solargis; http://globalsolaratlas.info) in the rain shadow. (e) Photograph of pilot irrigation trial in Murphys Creek using sulphate-rich water from Frasers waste rock stack on rough pasturage astride a flat, natural ridge (site indicated in b)
2 General Setting and Context of Study
2.1 Geology and Water
The Macraes deposit is a Cretaceous orogenic gold system within the Mesozoic lower greenschist facies Otago Schist belt of southern New Zealand (Fig. 1a, b; Craw & MacKenzie, 2016). Mining occurs primarily via open cut operations, with a small underground component, and waste rock is accumulated in large piles (> 100 m high) on the site (Fig. 1b). Processed ore, after gold extraction, is discarded into large tailings repositories that are impounded by walls of waste rock (Fig. 1b). The processed ore is rich in S-bearing minerals, such as pyrite (FeS2) and arsenopyrite (FeAsS), and their oxidised S-bearing derivatives. Oxidation of pyrite also occurs in waste piles from dumped rocks that have high surface areas due to blasting. The waste rock piles and tailings impoundment walls have been progressively revegetated with pasture grasses. As such, the rehabilitated mine areas merge with pastoral farming land leased to host grazing sheep and cattle (Fig. 1e).
Shallow groundwater emerges from the waste rock and tailings deposits to be intercepted in ponds and pumped back to the mine for further usage. However, some of this water is discharged downstream to surrounding catchments. Dissolved sulphate limits imposed by the local regulatory authority are directed at the principal usage of the mine water as stock water on farmland and are currently set at 1000 mg/L. Some discharge waters temporarily exceed this limit, but are rapidly diluted downstream.
Elevated levels of sulphate arise in two water sources in particular: Murphys Creek discharge originating from the Frasers waste rock stack, and the CJ seep from the wall of the tailings dam (Fig. 1b). These two waters are the focus of this study, in pilot field trials that used the water to interact with soils via farmland irrigation (Fig. 1b, e).
2.2 Soils and Vegetation
A thin layer of loess covers the mine area and principally forms a subsoil layer around 40-cm depth on lower relief, and as little as 4 cm on rocky areas. On narrow, flat spurs typical of the local area, Otago Schist is close to surface, with a shallow, colluvium cap to ~ 15–20-cm depth. Colluvium is made up of fine angular schist in a sandy-silty matrix derived from loess. Soils are predominantly Orthic Brown and Firm Brown Soils (Hewitt et al., 2021).
The predominant land use is relatively low productivity dryland farming (McNamara, 1992), on pastures dominated by exotic, low fertility grasses. Farmers apply lime to maintain the optimum pH of 5.5 to 6.25 required for the exotic legumes that sustain pasture grass production and also apply sulphur and phosphorus to enhance fertility. Natural vegetation is largely restricted to the numerous gullies that incise the Macraes landscape and around large outcrops or poorly drained hollows that afford some protection from grazing and fire used by farmers historically.
2.3 Topography and Climate
The mine site spans the drainage divides of several river catchments (Fig. 1b) on rolling uplands ~ 500 m above sea level and ~ 30 km inland from the east coast of the South Island (Fig. 1c, d). The mine site is located at the eastern edge of a prominent rain shadow that occurs in the lee of mountains on the western side of the island (Fig. 1c), and the rain shadow receives relatively high levels of solar irradiation (Fig. 1d). Prevailing westerly winds across the Tasman Sea cause high orographic rainfall in the western mountains (Fig. 1c) and foehn-style winds that regularly sweep across the rain shadow as far as the east coast. Hence, the Macraes mine site is almost constantly affected by drying winds with low relative humidity (Fig. 2a).
Background weather data for an 18-month period leading up to the irrigation trial, July 2018–December 2019, at Macraes mine site (automatic 10-min recording), and consequences of dry climate on surface waters pre-trial. (a) Wind speed and frequency. (b) Air temperature. (c) Daily cumulative rainfall. (d) Na and Cl concentrations in mine waters of this study (Murphys discharge; CJ seep) compared to regional evaporation of rain bearing marine aerosols. (e) Time series for sulphate concentrations in CJ Creek that receives the CJ seep, showing relative spikes in dry summer weather
The mine area has a temperate to semi-arid climate with warm summers and cool winters (Fig. 2b) and a mean annual temperature of 12 °C (Haffert et al., 2010). Precipitation in the area is ~ 600 mm/year, and this is primarily rainfall with minor snow events. Precipitation is spread throughout the year and is dominated by numerous small events (Fig. 2c). From rainfall, natural inputs of sulphur can be around 1–2 kg/ha/year (Boswell, 1994). Precipitation is offset by potential evaporation of ~ 700 mm/year that is enhanced by the persistent winds and high solar irradiation. Hence, the mine site and surrounding farm land have ongoing water shortages, especially during summer and autumn when drought conditions can arise. The mine obtains most of its water by pumping from a distant (> 20 km) river over an intervening ~ 400 m high hill, with some augmentation from groundwater intercepted in excavations. Background water sulphate contents are typically < 200 mg/L, and many sources have sulphate contents < 20 mg/L.
2.4 Water Compositions and Rationale for This Study
Surficial evaporation in the rain shadow locally causes development of Na-Cl salt evaporite deposits from marine aerosols in rain (Fig. 2d; Craw & Beckett, 2004; Druzbicka et al., 2015; Haffert et al., 2010). However, this saline effect is overshadowed in shallow groundwater at the Macraes mine by water–rock interaction reactions (Fig. 2d; Weightman et al., 2020). Both the water sources discussed in this study have dissolved sulphate of ~ 2500 mg/L (Fig. 3a). The Murphys discharge water flows at up to 10 L/s from beneath the waste rock stack into a large silt pond (Fig. 1b), from which water is recycled. The Murphys water has progressively evolved in composition over > 10 years through rapid dissolution of pyrite, calcite, and chlorite in the waste rock (Fig. 3a, b; Weightman et al., 2020). In contrast, the CJ seep contains ‘younger’ water (2 years of discharge) that discharges from the waste rock wall of the dam (Fig. 3a, b). This water accumulates in a small sump, from which it is periodically pumped back to tailings. Some overflow, typically with flow rates between 0.04 and 0.1 L/s, continues down CJ Creek to produce a periodic sulphate signal up to 1 km downstream (Fig. 2e). The composition of CJ seep water is different from that of the Murphys discharge with relatively higher Na and Ca from the ore processing system, and less influence of chlorite and calcite dissolution in waste rock (Figs. 2d and 3a, b; Weightman et al., 2020).
Compositions of source mine waters used in this study for Murphys discharge from the Frasers waste rock stack and the CJ seep from decant pond (Fig. 1b). (a) Time-series plot showing elevated sulphate in comparison to dissolved bicarbonate. Parallel EC scale is generalised. (b) Time-series data for bicarbonate, Ca, and Mg (enlarged section of a). (c) Mine water pH and sulphate compositions plotted on a geochemical model that indicates mineral saturation (from Geochemists Workbench software; Weightman et al., 2020)
Murphys discharge water has pH ~ 8, and this distinctly alkaline pH combined with the high dissolved Ca2+ and bicarbonate from waste rock has resulted in super-saturation with respect to Ca-carbonate minerals (Fig. 3c; Weightman et al., 2020). While dolomite is the most super-saturated mineral in these waters from a theoretical perspective (Fig. 3c), in reality spontaneous precipitation of a Ca-carbonate mineral, probably aragonite (CaCO3), is widespread and persistent along the discharge water channel, accompanied by only minor amounts (< 1%) of Ca-sulphate, probably gypsum (Fig. 4a; Weightman et al., 2020). High evaporation also leads to precipitation of Mg-sulphate as epsomite, as a short-lived precipitate rapidly redissolving in rain events (Fig. 4a).
Precipitate minerals that formed prior to irrigation trials. (a) Hard crust formed in water course at Murphy site. Spontaneous precipitate of super-saturated Ca-carbonate (aragonite, bottom and background), with a coating of Mg-sulphate (epsomite, top) precipitated during evaporational drying of creek bed material. (b) Epsomite coating on surficial debris below the CJ seep. (c) SEM image of principal evaporitic minerals derived from CJ seep water after complete drying in laboratory
CJ seep water has pH ~ 7/7.5 and is either saturated or slightly under-saturated with respect to Ca-carbonate minerals (Fig. 3c). Nevertheless, evaporation of CJ seep water leads to ephemeral precipitates of Mg-sulphate rather than Ca-sulphate (Fig. 4b; Weightman et al., 2020). In addition, minor Na-Mg sulphate (bloedite) and some NaCl and KCl can form after complete evaporation (Fig. 4c).
In the context of the above-described water compositions, the aim of this study was to lower the pH of both the Murphy discharge and CJ seep waters through soil interactions by distributing the waters on slightly acidic farm soils, with a typical range of pH 5–6. Theoretical modelling (Weightman et al., 2020) has predicted that acidification of the waters at pH 5–6, with associated evaporation, should lead to chemical saturation with respect to Ca- sulphate rather than Ca-carbonate and/or Mg-sulphate. Since Ca-sulphate is relatively insoluble, this process should effectively remove some of the dissolved sulphate from the water. At the same time, land irrigation could contribute to pastoral grass growth and paddock productivity, especially in dry periods. The dissolved constituents of the mine waters in this study are non-toxic to plants (Craw & Rufaut, 2017; Mains et al., 2006; Weightman et al., 2020) and even contain additional desirable plant nutrients (e.g. Mg, Ca, S, N), so pastoral grass growth may be further enhanced. To this end, we initially conducted a laboratory study, followed by pilot irrigation field trials in the Murphys Creek and CJ Creek catchments (Fig. 1b).
3 Methods
3.1 Soil Column Experiment
Water-soil interactions were simulated in a small experiment to generate data for modelling. Six PVC columns (7-cm diameter × 30-cm length) were individually filled with an intact core of topsoil with pasture, collected during an austral spring from a paddock adjacent to the CJ seep source water. The columns were placed in the Geology Department laboratory in a single row adjacent to windows that allowed a suitable light regime for plant growth. Two different water application rates were used to irrigate duplicate soil columns; 60 mL/day at a high rate (equivalent to ~ 12 mm/day, or three times the recommended irrigation rate for the mine environment) and 20 mL/day at a normal rate (equivalent to ~ 3 mm/day, the approximate recommended irrigation rate). Regional guidelines for pastoral irrigation in the Macraes area recommend an application rate of 3–4 mm/day of water (McIndoe et al., 2017). The source of water was the CJ seep (described above), collected on the same day as the soil wedges. Two additional soil columns acted as controls that received distilled water at 60 and 20 mL/day, respectively.
Water output was collected after 4 and 8 weeks of daily irrigation. The change in water pH from input to output water was measured to quantify acidification levels after soil interaction. Concentrations of SO4, Na, Mg, Ca, and Mn were analysed by ICP-MS to assess dilution/accumulation trends. Dismantled soil from each column was visually inspected for mineral precipitates, dried at 70° for 3 days, and re-examined under SEM–EDS for mineral identification.
3.2 Field Irrigation Trials
The irrigation trials utilised similar methodology to deliver water as occurred elsewhere at the mine to control dust. The main goal was to expose soils and plants to ongoing, high sulphate irrigation to obtain measurements related to mineral precipitation and beneficial or adverse effects. To deliver water to the two, 50 × 100 m trial plots, a 100-m length of PVC pipe (7-cm diameter) was set up near the source water at each site. Six 3-mm-wide holes, spaced 10 m apart, were drilled into the pipe wall to act as spray nodes. The amount of water distributed daily to the trial areas was a proportion of the water being pumped for wider mine recycling. Hence, irrigation application rates were variable throughout the study period and were quantified only generally.
At the CJ site, the three more proximal spray nodes to source water provided high to moderate flow rates and distributed ~ 15 L/day. The three more distal spray nodes received progressively lower application rates due to gravity effects on the water feed line and distributed ~ 3–4 L/day. The Murphys site received substantially higher flow, with applications rates at least an order of magnitude greater than CJ site (Fig. 1e). Regular daily irrigation was applied to the CJ site between 1 December 2019 and 31 May 2020, and from the 30 January 2020 to 31 May 2020 at the Murphy site. After irrigation ceased, the trial ran for a further 12 weeks until August 2020, to capture a winter period with natural precipitation only. Stock (cattle, sheep) were not excluded from trial areas and had full access during the study.
The baseline status of soils, pasture, and adjacent creek water was measured pre-irrigation in August–September 2019 (late winter/early spring). During the irrigation period, samples were also collected in February 2020 (late summer) and May 2020 (late autumn). There was one post-irrigation sampling period in August 2020 (winter). Control samples from an adjacent unirrigated area were taken during each sampling period to assess non-treatment seasonal conditions. Soils were sampled at two depth intervals, 4–30 cm (topsoil) and when present, 40–60 cm (subsoil), from random locations within a 5-m radius of each spray node. Each soil sample (volume weight c. 1 g/mL dried soil) represented a composite of three soil plugs (core diameter 5 cm), and 20–25 samples were collected each sampling month, dependent on subsoil access. Mixed foliage (grass, herbs, clover) was collected by gathering 30 g samples of composite fresh material (without any root material) from within soil sampling areas, with a total of 32 samples collected during the irrigation period. Post-irrigation foliage samples were not able to be collected due to low winter growth rates. Both of the trial areas were upslope from a nearby creek that had a history of mine water discharge and were included in the mine’s routine monitoring programme. Naive tributaries were specifically avoided during the study. To identify any penetration of irrigation water into riparian zones, repeat transects through each creek were conducted during soil sampling occasions to measure EC and pH every 10 riparian metres. Eight to ten creek water samples were also taken in each sampling month at both sites for dissolved sulphate analyses, dependent on water availability.
3.3 Analyses
Soil samples were used to repeat measure EC, pH, sulphate-sulphur, anion storage capacity, and moisture during the trial period. Total sulphur was also measured but only at the start and end of the study. Soil EC and pH values were obtained from 65 individual field samples in the Geology Department lab, using an Oakton field meter on a 2:1 fresh soil:distilled (100 g:50 mL) water slurry, stirred and left for 5 min before measuring. The meter was calibrated every 10th sample. Soil chemical analyses were performed on bulk samples by Hill Laboratories (NZ) using standard techniques as follows. Bulk soil samples consisted of pooling neighbouring pairs of spray nodes and control samples, to capture representative conditions, which gave 85 bulk samples. ‘Total’ sulphur nitric/hydrochloric digestion followed by ICP-OES, with 45 mg/kg detection limit, estimates the total pool of all forms of soil sulphur. Sulphate-sulphur (referred to herein as soil sulphate) potassium phosphate extraction followed by ion chromatography, with detection limit 1 mg/kg, measures readily available dissolved and adsorbed sulphate. Anion storage capacity by colorimetric analysis, with 3% detection limit, estimates sulphate retention in the soil. Moisture was calculated from the dry matter, to assess evaporation conditions and seasonal effects on sulphate test results. Foliage samples were bulked in a similar manner as soils, water washed, and dried before using nitric acid/hydrogen peroxide digestion followed by ICP-OES to determine sulphur uptake on 27 samples, with 0.02% detection limit, using Hill Laboratories (NZ). For creek water, dissolved sulphate concentrations were measured on 35 samples after filtration, using ion chromatography, with 0.5 mg/L detection limit (Hill Laboratories NZ). Levels of nitrogen and arsenic, components of the host rock, were tracked alongside sulphur species in soil, vegetation, and creek water, to regularly check adverse effluent impacts, but none were detected during the trial period and data are not presented.
Time-series data for changes in EC, pH, and dissolved sulphate are shown for creek water transects. Soil chemistry results are graphically displayed for sulphate, and data for anion storage capacity, moisture content, and total S presented as a supplementary file (Online Resource 1). The correlation between plant sulphur uptake and soil sulphate levels was calculated (from dry weight matter). Calibration graphs for water EC vs. dissolved sulphate and soil EC vs. soil sulphate were constructed to assess rapid monitoring techniques for sulphate loads. Graphs were drawn in Excel 10 and summary diagrams in the programme Freehand.
4 Results
4.1 Ion Concentrations During Lab Irrigation
The ‘high flow’ treatment produced a large amount of output water (> 100 mL) for chemical analyses, but only one of the ‘normal flow’ columns produced enough water for analyses at the end of the experiment (Week 8). Input water pH measurements dropped from pH 7.6 to between pH 4.1 and 5.5 in the outflow of soil columns, showing the influence of a more acidic soil environment on water interactions (Table 1). The outflow water from the high flow columns showed initial large decreases in ion concentration compared to the input water. Under ‘high flow’, Na, SO4, and Ca were all about 50% of the initial input concentration in the outflow at Week 4 (mid-way), Mg was 15–25% of the initial concentration, and Mn was 76–123%, respectively (Table 1). Initial soil moisture was 18–22%, and around 485 mL of pore water was estimated for each soil column. As irrigation time increased, the concentrations of most ions increased from the depleted concentrations at Week 4, to almost the levels of the input water. At Week 8 (end of experiment) in the ‘high flow’ samples, Na and SO4 were back up to 80–90% of the initial concentration, Mg was 43–55%, Ca was 103–117%, and K was 0–20% (Table 1). Some input from the soil was also expected in ion results, and control columns had 20–30 mg/L of Ca, Na, and sulphate in the output water.
Water output values from the ‘normal flow’ sample were similar to ‘high flow’ for Na, SO4, and Ca but not the remaining elements at Week 8 (Table 1). Pasture plants stayed green and healthy during the experiment but did not develop enough foliage biomass for chemical analyses of element uptake. Gypsum crystals were identified on the SEM–EDS around plant roots in irrigated but not control columns at the end of the experiment (Fig. 5a–c). No observations of precipitates were made on the surface of soil columns or plant foliage.
Experimental mineral precipitation from CJ seep waters. (a) Soil sample from CJ Creek area after laboratory irrigation experiment (see text), showing grass roots (white) with Ca-sulphate precipitates. (b) SEM image of plant root (left) with evaporative Ca-sulphate (right). (c) SEM image of evaporative Ca-sulphate crystals (probably gypsum) from roots
4.2 Sulphate Precipitation and Accumulation During Field Irrigation
Obvious effects of the irrigation regime developed early at the Murphy site. A widespread precipitate formed on plant foliage surrounding spray nodes within 4 weeks. The precipitate was dominated by Ca-carbonate, with minor Ca-sulphate crystals, and did not form on pasture in control areas (Fig. 6a–d). Plants with the precipitate coating were not observed to die off. Creek water monitoring also identified a rapid breach of the riparian zone by irrigation runoff. Flow rates and EC measurements increased above baseline levels after 4-week irrigation time (Fig. 6f). The latter were shown as a reliable proxy for dissolved sulphate effluent (Fig. 6e). Sulphate in creek water was 2000–2700 mg/L, EC was at 3500–4600 µS/cm, and pH at 6–7.2 during the irrigation period (Fig. 6f). After 12-week post-irrigation, sulphate and EC had decreased to 500–700 mg/L and 1000–1500 µS/cm, respectively, with pH remaining around 7 (Fig. 6f). Control waters around the Murphys trial area had sulphate < 100 mg/L and EC < 500 µS/cm.
Results of Murphys irrigation trial using Murphys discharge water (as in Fig. 1e). Precipitates of Ca-carbonate formed on outer leaf surface of (a) native tussock grass (b) on pasture grass and (c, d) on shrubs within the spray zone. Minor Ca-sulphate developed on shrub foliage in d. (e) Calibration of dissolved sulphate and electrical conductivity (EC) in Macraes mine waters. (f) Water pH and EC in a tributary of Murphys Creek downslope from the irrigation trial. Waste rock stack at tributary head prevented upstream water samples
At the CJ site, irrigation runoff also reached the margin of the creek from the ‘high’ flow spray nodes but not the more distal ‘low’ spray nodes (Fig. 7a). The creek initially had sulphate 400–800 mg/L, EC 1000–1300 µS/cm, and pH 6.5–7.5 downstream from the trial plot (Fig. 7b), with flow rates of c. 6–10 L/min. However, sulphate and EC became increasingly elevated upstream from the trial plot from independent sources of sulphate entering the creek, and this made monitoring of the irrigation trial more complex. The pH profiles also highlighted incursion of different water sources (Fig. 7b). Consequently, post-irrigation sulphate and EC responses were higher than during the irrigation period (Fig. 7b). Control waters around the CJ trial area had dissolved sulphate < 50 mg/L and EC < 250 µS/cm. Unlike the Murphy site, mineral precipitates at the CJ site were not observed during irrigation time. Instead, widespread Mg-sulphate formed on soils during the post-irrigation period. The formation of these sulphate minerals on soil after 12 weeks without irrigation formed from topsoil saturation and retention of residual sulphur available for evaporitic minerals (Fig. 8a, b).
Results of CJ irrigation trial using CJ seep water. (a) Drone photograph of the irrigated area at the end of the trial. Green patches indicate the irrigated portions. Brown patches indicate saturated soils. (b) CJ Creek pH and EC below the trial area. (c) Photograph of an irrigated area in the following spring (December 2020)
Photographs of field conditions showing (a) surface Mg-sulphate that formed post-irrigation at CJ, and (b) green, grazed pasture in latter stages of irrigation at Murphys site, with evaporative precipitate on irrigation pipe. (c) Soil sulphate in individual soil plugs from the Murphys site. (d) Soil sulphate at the CJ site. Underlined samples at CJ are from subsoil; all others are from topsoil. (e) Calibration of soil EC from field meter and soil sulphate from lab analyses. (f) Sulphur in pasture foliage at the two sites
The concentration of sulphate in soils under irrigation was observed to increase from baseline, and control values yet remained at less than 50% of the concentration contained in original source waters. Dilution was supplied by natural precipitation and inherent soil moisture content, which was between 13 and 24% in unirrigated samples (Online Resource 1). At the Murphys site, topsoil samples increased from baseline < 10 mg/kg (ranked as ‘low’ in interpretation values) to 400–700 mg/kg ( ‘very high’) after 16-week irrigation (Figs. 8c and 9). Topsoil samples at the CJ site had baseline < 15 mg/kg sulphate (ranked as ‘low’) that increased up to 1000 mg/kg (‘very high’) after 24-week irrigation (Figs. 8d and 9). Increased topsoil concentrations at CJ were successive over time so that sulphate was higher in May samples (average 790 mg/kg) than in February (average 500 mg/kg) (Fig. 8d). Topsoil samples located around the ‘high’ spray nodes at CJ had up to 1000 mg/kg sulphate and the ‘low’ nodes up to 800 mg/kg. A positive relationship (R2 = 0.71) was found between soil EC measured from a field meter and soil sulphate from lab analyses (Fig. 8e), indicating that field monitoring using EC on soil slurries was a reliable proxy for changes in soil sulphate concentrations (see also Fig. 6c for a similar relationship with water). Subsoils were difficult to locate at the Murphy site (with bedrock close to surface), but samples collected also showed impacts of irrigation water, with increased sulphate of 400–500 mg/kg compared to < 10 mg/kg in baseline and control samples (Fig. 9). At the CJ site, with deeper subsoils, sulphate ranged between 200 and 400 mg/kg in samples taken, compared to < 25 mg/kg in baseline and control samples (Fig. 9). During the post-irrigation winter period, soil sulphate concentrations were seen to start to trend downwards (Fig. 8c, d). The lowering of soil sulphate levels was most apparent at the Murphys site, where topsoil measurements decreased on average by 50%, from 600 to 250 mg/kg respectively (Fig. 8c).
An indication of total sulphur concentrations was obtained to assess a greater pool of S in soils, alongside immediately available S from the sulphate analyses (Online Resource 1). Total S increased from being ranked as ‘very low’ at < 400 mg/kg at both sites to ‘high’ at the CJ site and ‘low/medium’ at Murphys, with average values at 1200 mg/kg and 600 mg/kg during irrigation, respectively. Higher maximum values for total sulphur at CJ are at least partially explained by a secondary, unplanned source of S in the form of fertiliser (3% P, 15% Ca, 5% S) applied to pasture inadvertently by the farmer, just prior to the field trial starting. The presence of fertiliser may also explain the deviation of some CJ samples from the soil analysis calibration plot in Fig. 8e.
4.3 Plant Performance Under Field Irrigation
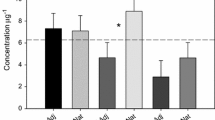
Mixed pasture plants within the irrigation halo but outside of the highly saturated areas were greener than control areas and showed no signs of die back or foliage deficiencies during the trial period (Fig. 7c). Plants in the direct line of the three higher-flow spray nodes became waterlogged and died back locally (Fig. 7a). Maximum grass die back occurred after 6–7 months as 1-m-wide strips up to approx. 20 m downslope (Fig. 7a; brown strips). Stock use of irrigated areas caused further damage to saturated grass and soil by trampling. Soil moisture was maintained around 26–30% during irrigation time compared to < 20% in non-irrigated areas (Online Resource 1). Stock appeared to favour the irrigation areas, with regular grazing observed. Due to the presence of stock, pasture performance in terms of biomass and height growth were not measured. Pasture foliage was instead analysed for sulphur uptake during the summer-autumn growth season. A positive trend developed between soil sulphate and plant sulphur (r = 0.80, R2 = 0.64) within the trial period (Fig. 8f). Irrigated pasture plants had 1–2.2% sulphur in their leaves compared to < 0.5% in baseline and control plants at the CJ site (Fig. 8f). The CJ site’s elevated pasture sulphur content probably reflected fertiliser inadvertently applied pre-trial (above). Plant sulphur % values were converted into mg/kg dry weight for standardised comparison with levels of soil sulphate (Fig. 9). By the end of the irrigation period (May), foliage sulphur concentrations had risen to be 13–14 times higher than in corresponding topsoil (Fig. 9).
5 Discussion
5.1 Mineral Precipitation from Mine Water Irrigation
In this study, the potential use of high-sulphate mine water (c. 2500 mg/L) for future irrigation on surrounding farm land within the Macraes mine boundaries was investigated with initial pilot trials. Theoretical modelling in our earlier study of two discharge waters sourced from different waste rock constructions suggested that acidification of the mine’s circumneutral waters (pH 7–8) would favour chemical saturation of Ca-sulphate (forming gypsum CaSO4.2H2O) over Ca-carbonate (forming aragonite CaCO3) or Mg-sulphate (forming epsomite MgSO4), across a realistic evaporation gradient for mine site conditions, to enhance removal of dissolved sulphate (Weightman et al., 2020). Gypsum precipitation is more desirable over epsomite due to lower solubility (2 g/L vs. 731 g/L (Elvira-León et al., 2016), meaning it will be more resistant to re-entering solution once precipitated. Under our model simulations, between 25 and 50% of water would need to evaporate during an irrigation regime to induce gypsum formation under pH 5–6 conditions. If these theoretical processes occurred in reality, via use of water interactions with mine site’s slightly acidic soils (pH5–6), we expected gypsum to form in upper soil layers and/or on the soil surface. Laboratory interactions using CJ seep water as the model showed significant acidification of output water (by 2–3 pH units) from soil columns occurred after 4 weeks. The 8-week experiment resulted in gypsum forming on the root systems of mine water treated pasture and not in control columns with distilled water (Fig. 5). Similar mineral salt accumulation in root zones has been observed elsewhere (Bennett et al., 2009), with no apparent negative effects recorded from gypsum on roots of growing plants (Syslo et al., 1988). Zilberbrand (1996) analysed the effect that gypsum had on carbonate attenuation and demonstrated that crystallisation in the root zone was responsible for decreasing HCO3 and Ca in groundwater.
In contrast to findings in our laboratory experiment, the small-scale field trials did not produce any observations of gypsum in soils irrigated with mine water at two study sites. It is possible small amounts formed, were redissolved and missed by visual observations, or seasonal effects in rain and plant growth were overlooked but we largely attribute a lack of field gypsum to over-irrigation. The unregulated water application rates used caused soil saturation rather than evaporation and resulted in the alkaline source water overwhelming initial pH acidification effects from the soils. Hence, the environmental requirements of our theoretical model were not well met in the field trial. Gypsum’s absence may be partially a result of chemical over-saturation, without sufficient biologically mediated precipitation processes (cf. Bowell, 2004). The lack of gypsum during the field study is in strong contrast to the Macraes mine tailings system, where gypsum precipitation along water flow paths under pH 6–7 partially attenuated concentrations of dissolved sulphate that reached as high as 8000 mg/L (Craw, 2003; Craw & Pope, 2017). In contrast, we found that epsomite precipitation occurred more readily than gypsum in field soils interacting with mine water (Fig. 8a). In a recent study of sand mining pit water, Figueiredo et al. (2020) also determined that the pH of irrigation water affected soil pH, indicating that making use of pH conditions in soil has a potentially temporal component under irrigation regimes, which was not considered by Weightman et al. (2020) when modelling geochemical outcomes for the Macraes mine water.
Figure 10 summarises contrasts in chemical parameters and pathways for mineral precipitation under different pH conditions observed to date during this study. Discharging water at the Murphys site readily produces Mg-sulphate via evaporation of ephemeral in situ seeps and Ca-carbonate spontaneously in aqueous settings, after 10-year bedrock-water interactions. When this water is diverted for pastoral irrigation, equal parts Mg-sulphate and Ca-carbonate, with minor Ca-sulphate, form on terrestrial surfaces via evaporation. Specifically, vegetation under irrigation with Murphys source water develops Ca-carbonate on above-ground foliage and potentially minor Ca-sulphate around root zones, inferred from lab simulations (Fig. 10). At the CJ site, in situ seeps produce mostly Mg-sulphate via evaporation, with some Na-Mg-sulphate and minor Ca-carbonate, after 2-year bedrock-tailings-water interactions. No aqueous precipitates were observed to form in the creek. When diverted for irrigation, Mg-sulphate still predominantly forms on terrestrial surfaces via evaporation. For vegetation, no precipitates were observed on above-ground foliage, but Ca-sulphate formed around plant roots in the lab experiment (Fig. 10).
5.2 Dilution and Leaching of Sulphate in Irrigated Soils
An absence of significant amounts of sulphate-bearing minerals in the field trials means that the changes observed in sulphate concentrations from source water to the soil profile are explained primarily by dilution from soil pore water, rain, and leaching. At the end of the 8-month trial, topsoil sulphate values were at 35–40% of source water input at the CJ site and 20–30% at Murphys, respectively (Fig. 9). The amount of sulphate in topsoil samples was 1500–2000 mg/kg lower than sulphate levels in source water, indicating significant reduction in concentrations. Subsoil data was most robust at the CJ site and showed a further decrease of 350–600 mg/kg sulphate, at around 60-cm depth, respectively (Fig. 9). Sulphate accumulation in the subsoil, to levels well above baseline and control values, also reflected over-irrigation conditions where water was driven downwards from saturated topsoils. The influence of sulphate in soils was detected up to a distance of 50 m downhill from the high flow spray nodes, to similar levels to those at 5 m downhill, indicating the soil data in Figs. 8 and 9 were representative of values over a larger spatial scale. Leaching processes depend on some soil characteristics such as organic matter and accompanying cations (Cichota et al., 2007; Edmeades et al., 2005; Geurts et al., 2009; Goh & Nguyen, 1997), but these parameters were not measured in this study.
Leaching losses of the sulphate anion are associated with the soil’s anion storage capacity (ASC) that was quantified in this study (cf. Blakemore et al., 1987).
For this study, measured ASC showed values ranked at the lower end, 30–40%, indicating high leaching losses for sulphate at both study sites (Online Resource 1). Hence, contamination of sulphate beyond the topsoil layer, into subsoils and adjacent creeks, can be explained by over-irrigation causing surface runoff and/or downward water percolation, a natural soil environment with high potential leaching potential, shallow or absent subsoils, and highly fractured surfaces on bedrock. In addition, the irrigations trials did not cover available areas evenly, and this irregular water distribution contributed to localised water channelling and localised under-irrigation. Soil columns in the lab trial under simulated ‘normal flow’ irrigation rates (based on 3–4 mm/ha/day) produced limited output water over 30-cm depth, highlighting appropriate water application rates as an important consideration for future irrigation work near the mine. This could be especially significant for irrigation of rehabilitated land at the mine site itself, as the engineered soil cover is typically porous and stony with less fine-grained clay components and organic matter (Craw & Rufaut, 2017) than natural soils in surrounding farmland.
A study of spray-irrigated pastures in North Canterbury, another region of New Zealand prone to drought, showed up to 60% of water applied can be lost by leaching beyond the rooting zone of pasture plants (Boswell, 1994). Associated leaching of S can exceed 40 kg/ha/year in dairy farming settings (Edmeades et al., 2005; Goh & Nguyen, 1997). A 10-year irrigation study in Chile that used creek water with dissolved sulphate levels at 1400 mg/L from a copper mine for alfalfa irrigation found that no sulphate was retained in the measured soil because it was rapidly leached (Moreno et al., 2009). Some studies have demonstrated lower sulphate concentrations from microbial sulphate reducing processes in saturated soils (e.g. Bowell, 2004; Geurts et al., 2009). However, Weightman et al. (2020) found no evidence for such reduction at the Macraes site, and our observations from the present study suggest that essentially all S remains as sulphate.
5.3 Benefits of Pastoral Irrigation from Mine Water
In this study, mixed pasture under the influence of mine water irrigation stayed green and showed no visual adverse effects in foliage colour, size, or shape (e.g. pale, yellow leaves in new growth) where aerobic conditions were maintained. The uptake of sulphate in plants was varied yet showed a strong response at each study site, 0.7–2.3% S, compared to typical mixed pasture of 0.3–0.4% S not exposed to high sulphate water. The concentration of foliage sulphur was measured at 13–14 times higher than levels of sulphate in topsoil samples (Fig. 9). A significant plant response was expected from baseline measures of total sulphur in soil, which indicated a S deficiency (< 600 total S mg/kg). Farmers in the region apply fertiliser containing c. 5% S to overcome soil deficiencies, as occurred in an uncontrolled incident during the CJ site field trial. Interestingly, Ca and P are also added to pasture fertilisers at Macraes. This means that the wider chemical profile of mine water used in this study, with elevated S, Ca, Mg, N from waste rock-water interactions, has potential to supply plant nutrients currently obtained via fertiliser application, except P that is available at naturally low levels in the host rock (Craw & Rufaut, 2017). Maximum pasture yield in New Zealand requires annual S addition of ~ 100 kg/hectare (Edmeades et al., 2005), and this equivalent level of addition was met at the CJ site after 6 months of mine water irrigation (Online Resource 1). Although the volumes of equivalent plant nutrients that could be added through irrigation of waste rock water are much larger than would be typically applied in farm management, there are potentially multiple benefits of enhanced plant nutrient availability and soil moisture from reusing mine’s waste rock discharge water. Provided the water application rates and runoff volumes can be managed, this approach warrants further investigations.
6 Conclusions
A pilot investigation into evaporation and acidification (from pH 7–8 to 5–6) of waste rock mine water to enhance sulphate-bearing mineral precipitation, especially gypsum (Ca-sulphate), as a potential solution for lowering dissolved sulphate loads (c. 2500 mg/L) in discharge at Macraes Gold Mine had mixed results. Two different water sources, Murphys discharge and CJ seep, had distinct chemical profiles from rock-water interactions that favoured evaporitic mineral formation in the semi-arid mine site setting under geochemical modelling. A lab-based irrigation simulation was shown to produce gypsum (Ca-sulphate) in pasture root zones treated with mine water, and soil columns had output water pH at 2–3 units less than input water. Initial observations of sulphate attenuation were short-lived and attributed primarily to dilution from use of field soil samples during the experiment. Subsequent field-based irrigation trials during summer-autumn failed to closely simulate ideal model environmental conditions regarding application rates, evaporation, and pH to optimise larger scale gypsum formation in slightly acidic topsoils. Over-use of daily water, combined with uneven application to the land, led to soils becoming locally saturated and more alkaline (pH 6.5–7) over time, and undesirable breaches into nearby riparian zones and subsoils were recorded.
Contrary to model predictions, in reality epsomite (Mg-sulphate) and aragonite (Ca-carbonate) were observed more readily in the field and in greater abundance in soils interacting with irrigation mine water than the predicted gypsum (Fig. 10). Only minor gypsum co-occurred with the dominant minerals, epsomite and aragonite (Ca-carbonate) at the Murphy site, and epsomite with bloedite (Na-Mg-sulphate) at the CJ site. Gypsum’s general absence from the field study and observed association with vegetation surfaces raise the possibility that its precipitation is driven by biological mediated processes rather than chemical saturation alone.
Repeat measurements from soil-mine water interactions showed a pronounced increase in sulphate concentrations in irrigated topsoil, up to 700–1000 mg/kg over 4–6 months, compared to baseline values of < 15 mg/kg. The soils used had a naturally high leaching capacity because they are shallow, stony, and there were some preferential water flow paths. Consequently, sulphate was shown to accumulate in the subsoil layer, up to 400–500 mg/kg. After 12 weeks post-irrigation, winter soil sulphate concentrations appeared to be declining, and this continues to be monitored at the study sites. The above trends in soil sulphate analyses were also apparent in less formal monitoring techniques that used a field meter and soil slurries to measure electrical conductivity. Hence, soil EC values were a suitable proxy for assessing soil sulphate levels at the Macraes mine site and provide a cost-effective method for monitoring this type of irrigation activity.
A strong uptake of sulphate from irrigation water was seen in mixed pasture plants, and maximum yield requirements of 900 mg/kg of soil sulphate were approached in the field trial. Deeper rooted grasses may also have accessed some sulphate in the subsoil c. 40-cm depth. The CJ site had higher plant uptake and higher soil sulphate concentrations than the Murphys site, which was attributed to the additive effects from fertiliser applied by the farmer at this site.
The mine source waters are enriched in Ca, N, Mg, as well as S, and so irrigation with mine water has the potential dual benefit of providing major plant nutrients and moisture to enhance farm productivity. However, appropriate irrigation application rates need to be managed, possibly with some localised engineering to avoid overland channelling of water flow. If a balance can be met between appropriate sprinkler irrigation, ground water monitoring, and high supply of source water at the mine, we conclude pastoral irrigation is a viable approach to manage dissolved sulphate loads in discharging waters from waste rock stacks.
Data Availability
All data relevant to this study are included within this paper and within cited references. A table of supplementary data is supplied.
References
Bennett, S. J., Barrett-Lennard, E. G., & Colmer, T. D. (2009). Salinity and waterlogging as constraints to saltland pasture production: A review. Agriculture, Ecosystems and Environment, 129, 349–360.
Blakemore, L.C., Searke, P.L., & Daly, B.K. (1987). Methods for chemical analysis of soils. New Zealand. Soil Bureau Scientific Report 80. Department of Science and Industrial Research, New Zealand.
Boswell, C. C. (1994). Fertiliser sulphur for North Canterbury pastures. Proceedings of the New Zealand Grassland Association, 56, 17–22.
Bowell, R.J. (2004). A review of sulphate removal options for mine waters. In: Jarvis, A.P., Dudgeon, B.A., and Younger, P.L. (Eds). Mine Water 004: Proceedings of the International Mine Water Association Symposium 2, pp 75–91.
Canovas, C. R., Olias, M., Nieto, J. M., & Galvan, L. (2010). Wash-out processes of evaporitic sulfate salts in the Tinto river: Hydrogeochemical evolution and environmental impact. Applied Geochemistry, 25, 288–311.
Cichota, R., Vogeler, I., Bolan, N. S., & Clothier, B. (2007). Cation influence on sulfate leaching in allophanic soils. Australian Journal of Soil Research, 45, 49–54.
Craw, D. (2003). Geochemical changes in mine tailings during a transition to pressure–oxidation process discharge, Macraes Mine, New Zealand. Journal of Geochemical Exploration., 80, 81–94.
Craw, D., & Beckett, S. (2004). Water and sediment chemistry of Sutton Salt Lake, east Otago, New Zealand. New Zealand Journal of Marine and Freshwater Research, 38, 315–328.
Craw, D., & Pope, J. (2017). Time-series monitoring of water-rock interactions in mine wastes, Macraes gold mine, New Zealand. New Zealand Journal of Geology & Geophysics, 60, 159–175.
Craw, D., & Rufaut, C. (2017). Geochemical and mineralogical controls on mine tailings rehabilitation and vegetation, Otago Schist, New Zealand. New Zealand Journal of Geology and Geophysics, 60, 176–187.
Craw, D., MacKenzie, D. (2016). Macraes gold deposit, New Zealand. SpringerBriefs in World Mineral Deposits. ISBN 978–3–319–35158–2, 130 pp.
Dold, B. (2017). Acid rock drainage prediction: A critical review. Journal of Geochemical Exploration, 172, 120–132.
Druzbicka, J., Rufaut, C., & Craw, D. (2015). Evaporative mine water controls on natural revegetation of placer gold mines, southern New Zealand. Mine Water & Environment, 34, 375–387.
Edmeades, D. C., Thornold, B. S., & Roberts, A. J. C. (2005). The diagnosis and correction of sulfur deficiency and management of sulfur requirements in New Zealand pastures: A review. Australian Journal of Experimental Agriculture, 45, 1205–1223.
Elghali, A., Benzaazoua, M., Bouzahzah, H., Bussiere, B., & Villarraga-Gomez, H. (2018). Determination of the available acid-generating potential of waste rock, part I: Mineralogical approach. Applied Geochemistry, 99, 31–41.
Elvira-León, J., Chimenos, J., Isábal, C., Monton, J., Formosa, J., & Haurie, L. (2016). Epsomite as flame retardant treatment for wood: Preliminary study. Journal of Construction & Building Materials, 126, 936–942.
Fernando, W. A. M., Ilankoon, I. M. S. K., Syed, T. H., & Yellishetty, M. (2018). Challenges and opportunities in the removal of sulfate ions in contaminated mine water: A review. Mineral Engineering, 117, 74–90.
Figueiredo, F., Leite, G. D., Donagemma, G. K., Teixeira, P. C., Donagemma, R. A., do Canto, A. C. B., de Carvalho Balieiro, F., Martins, G. S., & da Silva, L. D. B. (2020). The impact of water from sand pit lakes on plant growth, soil, and leachate. Water Air Soil Pollution, 231, 268.
Geurts, J. J. M., Sarneel, J. M., Willers, B. J. C., Roelofs, J. G. M., Verhoven, J. T. A., & Lamers, L. P. M. (2009). Interacting effects of sulphate pollution, sulphide toxicity, and eutrophication on vegetation development in fens: A mesocosm experiment. Environmental Pollution, 157, 2072–2081.
Goh, K. M., & Nguyen, M. L. (1997). Estimating net annual soil sulfur mineralisation in New Zealand grazed pastures using mass balance models. Australian Journal of Agricultural Research, 48, 477–484.
Haffert, L., Craw, D., & Pope, J. (2010). Climatic and compositional controls on secondary arsenic mineral formation in high-arsenic mine wastes, South Island, New Zealand. New Zealand Journal of Geology & Geophysics, 53, 91–101.
Hammarstrom, J. M., Seal, R. R., II., Meier, A. L., & Kornfeld, J. M. (2005). Secondary sulfate minerals associated with acid drainage in the eastern US: Recycling of metals and acidity in surficial environments. Chemical Geology, 215, 407–431.
Hewitt AE, Balks MR, Lowe DJ. (2021). The soils of Aotearoa New Zealand. World Book Series, Springer International Publishing, 332 pp. ISBN 978–3–030–64763–6.
Hudson-Edwards, K. A., Schell, C., & Macklin, M. G. (1999). Mineralogy and geochemistry of alluvium contaminated by metal mining in the Rio Tinto area, southwest Spain. Applied Geochemistry, 14, 1015–1030.
Jamieson, H. E., Walker, S. R., & Parsons, M. B. (2015). Mineralogical characterization of mine waste. Applied Geochemistry, 57, 85–105.
Jerz, J. K., & Rimstidt, J. D. (2003). Efflorescent sulfate minerals: Paragenesis, relative stability and environmental impact. American Mineralogy, 88, 1919–1932.
Lottermoser, B. (2010). Mine wastes: Characterization, treatment and environmental impacts (p. 400). Springer.
Mains, D., Craw, D., Rufaut, C. G., & Smith, C. M. S. (2006). Phytostabilisation of gold mine tailings, New Zealand. Part 1: Plant establishment in an alkaline substrate. International Journal of Phytoremediation, 8, 131–147.
McIndoe, I., Brown, P., Rajanayaka, C., Birendra,K.C., (2017) Guidelines for reasonable irrigation water requirements in the Otago region. Otago Regional Council, Volume 2. Aqualinc Research Limited, 43 pp.
McNamara, R. M. (1992). Seasonal distribution of pasture production in North and East Otago downlands. New Zealand Journal of Agricultural Research, 35, 163–169.
Moreno, P., Aral, H., & Vecchio-Sadus, A. (2009). Environmental impact and toxicology of sulphate. Enviromine 2009 Conference Paper, Santiago, Chile.
NIWA. The New Zealand National Climate Database. (2012). Available online: http://cliflo.niwa.co.nz/ (accessed on 4 February 2020)
Parbhakar-Fox, A., & Lottermoser, B. G. (2015). A critical review of acid rock drainage prediction methods and practices. Mineral Engineering, 82, 107–124.
Prasad, B., Sudkip, M., Mondal, M. R., & Kumar Singh, K. K. (2021). Evaluation of treatment techniques for utilising acid mine water in agriculture. Water, Air & Soil Pollution, 232, 190.
Smith, L. J., Blowes, D. W., Jambor, J. L., Sego, D. C., & Neuner, M. (2013). The Diavik Waste Rock Project: Particle size distribution and sulfur characteristics of low-sulfide waste rock. Applied Geochemistry, 36, 210–221.
Syslo, S., Myhre, D., & Harris, W. (1988). Calcium sulfate crystallization along citrus root channels in a Florida soil exhibiting acid sulfate properties. Journal of Soil Science, 145(2), 126–134.
Tolonen, E.-T., Ramo, J., & Lassi, U. (2015). The effect of magnesium on partial sulfate removal from mine water as gypsum. Journal of Environmental Management, 159, 143–146.
Tolonen, E.-T., Hu, T., Ramo, J., & Lassi, U. (2016). The removal of sulfate from mine water by precipitation as ettringite and the utilisation of the precipitate as a sorbent for arsenate removal. Journal of Environmental Management, 181, 856–862.
Weightman, E., Craw, D., Rufaut, C. G., Kerr, G., & Scott, J. (2020). Chemical evolution and evaporation of shallow groundwaters discharging from a gold mine, southern New Zealand. Applied Geochemistry, 122, 1–15.
Zilberbrand, M. (1996). The effect of carbonates and gypsum precipitation in the root zone on the chemical composition of groundwater. Journal of Hydrology, 174, 5–22.
Acknowledgements
We especially appreciate the logistical support and useful discussions with Scott Mossman and Gavin Lee at the Macraes mine. SEM work at the Otago Micro and Nano Scale Imaging Centre (OMNI), University of Otago, was ably supported by Marianne Negrini. A thorough and constructive review by an anonymous referee substantially improved the presentation of the manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This research was funded by Oceana Gold Ltd and University of Otago.
Author information
Authors and Affiliations
Contributions
The lead and corresponding authors are responsible for correctness of the statements provided in the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rufaut, C., Weightman, E., Craw, D. et al. Potential for Pastoral Irrigation Using Sulphate-Rich Waters at Macraes Gold Mine, Southern New Zealand. Water Air Soil Pollut 233, 391 (2022). https://doi.org/10.1007/s11270-022-05838-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-022-05838-9