Abstract
Urban areas may support high densities of wild carnivores, and pathogens can strongly influence carnivore populations. Red foxes (Vulpes vulpes) are hosts of sarcoptic mange (Sarcoptes scabiei), which infects numerous species, and transmission can be density dependent. In Great Britain, urban red foxes (Vulpes vulpes) have recently increased in population density and undergone range expansions. Here we investigate corresponding changes in urban fox mange prevalence. We predicted a higher prevalence closer to historic epi/enzootics and lower prevalence where urban features reduce fox density and movements, i.e. large areas of public green space, and fragmented habitat, as measured by road length and urban perimeter shape complexity. We visually assessed mange symptoms from georeferenced images of urban foxes submitted online by the public, thus surveying private land on a national scale. We measured the proportion of foxes apparently showing mange and used SATSCAN to identify spatial clusters of high infection risk. Landscape features were extracted from urban layers in GIS to determine associations. Although mange was widespread, we identified a single cluster of high prevalence (37.1%) in Northwest and Central England, which exceeded double mean prevalence overall (15.1%) and mirrors the northward expansion of urban fox distribution. Prevalence was positively correlated with perimeter shape complexity and negatively correlated with distance to the nearest city with mange, although the latter association was weak. Our findings show that citizen science can effectively monitor diseases with highly visible symptoms and suggest that fox movements are influential in explaining spatial patterns of prevalence.
Similar content being viewed by others
Introduction
Land-use changes associated with increasing global urbanization typically lead to detrimental effects on biodiversity (McKinney 2008). However, some wild species such as the red fox (Vulpes vulpes) are urban exploiters and may reach higher population densities in towns and cities than in rural areas (Smith and Wilkinson 2003; Baker and Harris 2007; Soulsbury and White 2015). In Great Britain (hereafter termed Britain) urban fox distribution has expanded within the last 30 years (Scott et al. 2014) with an increase in social group density in some cities that can plausibly be extrapolated nationwide (Scott et al. 2018). Rising wildlife densities can heighten the risk of transmission of pathogens, which can affect wild host populations dynamics (Tompkins et al. 2002), increase transmission risk to other host species, and create knock-on-effects on sympatric predators and prey (Lindström et al. 1994). Further, spill-over of disease to domestic animals and humans can occur (e.g. Menzano et al. 2004) therefore it is essential that infectious disease processes in urban wildlife populations are adequately understood.
Red foxes are major hosts of several serious diseases in Europe and globally, some of which are increasing in prevalence (Baker and Harris 2007; Kauhala et al. 2016). Significant veterinary pathogens carried by foxes globally include canine distemper virus (Nouvellet et al. 2013), heartworm (Dirofilaria immitis) (Tolnai et al. 2014) and the lungworm Angiostrongylus vasorum (Morgan et al. 2008; Taylor et al. 2015). Zoonoses transmitted by foxes include rabies (Lyssavirus sp.) (Smith and Wilkinson 2003; Smith et al. 2003; Singer and Smith 2012), canine roundworm Toxocara canis, the tapeworm Echinococcus mutlilocularis (Brochier et al. 2007) and the burrowing mite Sarcoptes scabiei. Variants of the latter cause sarcoptic mange in non-human mammals and scabies in humans (Davidson et al. 2008; Plumer et al. 2014; Pisano et al. 2019). Of the pathogens listed, S. scabiei and A. vasorum are endemic in Britain, and rabies and E. multilocularis have the potential to (re)emerge. Sarcoptic mange (hereafter termed mange) is particularly well documented in urban fox populations, (e.g. Newman et al. 2003; Soulsbury et al. 2007; Plumer et al. 2014) and although this may partially reflect the high visibility of both the symptoms and the host species, the disease is nonetheless considered to play a significant role in long-term fox population dynamics (Smith and Wilkinson 2003; Soulsbury et al. 2007). Mange has also been shown to cause negative effects on biodiversity globally (Daszak et al. 2000) including disease-induced mortality of endangered carnivores (e.g. Gakuya et al. 2012; Cypher et al. 2017).
The mange mite is a neglected, re-emerging and widely distributed ectoparasite that infects over 100 mammalian species (Bornstein et al. 2001; Devenish-Nelson et al. 2014; Carricondo-Sanchez et al. 2017). It is highly genetically variable with numerous strains infecting different species including S. scabiei var. vulpes (fox), var. canis (canids), var. rubicaprae (Northern chamois Rubicapra rubicapra) and var. hominis (humans) (Alasaad et al. 2012; Arlian and Morgan 2017). Symptoms include hyperkeratosis, erythema, and intense pruritus with encrusted dermal lesions that can become infected with bacteria and yeast, potentially leading to severe condition loss and emaciation (Alasaad et al. 2012; Niedringhaus et al. 2019). Mange is highly contagious and although symptom severity is variable and not always fatal (Gehrt et al. 2010), it can lead to host fatality in naïve populations in 2–4 months without treatment (Newman et al. 2002). Immune responses of wild mammals to sarcoptic mange are not well-understood (e.g. Bornstein et al. 2001) although the probability of mortality is known to be affected by season, host immune function, co-infections and nutritional status (Nimmervoll et al. 2013; Haas et al. 2018). Mange is transmitted between infected and susceptible individuals and heavy infections can accumulate rapidly (Bornstein et al. 2006) especially in naïve populations. Direct contact is perceived as the primary mode of infection (Devenish-Nelson et al. 2014; Arlian and Morgan 2017) but indirect transmission via fomites (infective objects or materials) also occurs, as all life-cycle stages are viable in the environment for up to 20 days (Arlian and Morgan 2017). Further, any form of contact that leads to transmission is likely to depend on degree of sociality and space use in the host species (Montecino-Latorre et al. 2019).
In Britain, the city of Bristol in Southwest England notably bucks the trend of progressively increasing fox density over the last 30 years due to a mange epizootic in 1994 that resulted in a population reduction of >95% (Soulsbury et al. 2007). This was apparently exacerbated by extremely high fox density at the time combined with previous lack of exposure. Consequent rapid spread to other, naive urban populations across Britain resulted in the presence of enzootic infection in most conurbations by 2001 (Soulsbury et al. 2007). Meanwhile, mange-related mortality in the depleted Bristol fox population remained high, indicating frequency (density independent) transmission (Soulsbury et al. 2007; Devenish-Nelson et al. 2014). Similarly, Carricondo-Sanchez et al. (2017) found evidence for frequency-dependent transmission in Norway. However, in Europe and globally, studies report both positive (e.g. Uraguchi et al. 2014) and negative (Gortazar et al. 1998) correlations between mange prevalence and fox density, with the latter also negatively predicting rate of spread (Lindström and Morner 1985). Clearly, the links between prevalence, spread and population density are complex and context specific (e.g. Niedringhaus et al. 2019) although historic infection is likely to play a role.
A definitive diagnosis of mange requires microscopic identification of mites from skin scraping/biopsy or more commonly by sero-diagnosis using an Enzyme-linked immunosorbent assay (ELISA) test supplemented by Western blot (e.g. Fuchs et al. 2016). These procedures, however, are invasive and logistically challenging and require animal capture, reducing the scope for quantifying prevalence over large areas. As mange typically manifests as lesions/alopecia in infected animals, the use of photographs is a promising alternative. Indeed, Carricondo-Sanchez et al. (2017) non-invasively documented spatio-temporal patterns of mange in foxes at the regional scale in Norway by visually determining visible lesions/alopecia from remote camera ‘trapping’ images. Using this approach, they found evidence of enzootic infection via small localized outbreaks, and clusters of high apparent prevalence in peri-urban areas in winter.
Mange prevalence in Britain was investigated in 2001 via questionnaire surveys of the public and animal welfare organizations (Soulsbury et al. 2007). The study found a broad distribution overall, with a peak in prevalence in Central and Southern England, mirroring urban fox distribution at the time, which was in the process of expanding from the South of England to the North and East (Scott et al. 2014, 2018). However, participant perception was liable to have biased the results, and both prevalence and spatial extent may have altered since 2001 following changes in fox distribution and density. Fox movements, particularly juvenile dispersal, are believed to strongly influence mange spread (e.g. Devenish-Nelson et al. 2014). Further, genetic studies reveal high microsatellite diversity and little isolation by distance in fox populations in Britain and parts of continental Europe e.g. Poland (Teacher et al. 2011; Atterby et al. 2015) indicating that there are few barriers to movement. Minimal genetic differentiation has been found between UK urban and rural populations (Atterby et al. 2015) and very long-distance movements have been documented for individual urban foxes in Britain (e.g. Tolhurst et al. 2016). In striking contrast, differentiation in genetic structuring within and between urban fox populations was documented in the greater Zurich area, Switzerland where both natural barriers (e.g. rivers) and varying anthropogenic infrastructural complexity (e.g. of building density and dimensions) created fragmented subpopulations with limited dispersal between them (DeCandia et al. 2018). Reduced mange spread might be expected to result from correspondingly minimal (direct or indirect) contact between susceptible and infected individuals. This could occur in high density populations where these are distributed within small, stable and contiguous territories with limited movements between them (e.g. White et al. 1996; Baker et al. 2000).
Field investigations of urban foxes on a large scale are constrained by private land ownership and associated barriers to access. However, human populations in urban areas are intrinsically high, presenting opportunities for harnessing observations of engaged citizens to collect large datasets at the regional scale, i.e. citizen science. In this way, access issues can be overcome. Indeed, citizen science data have successfully been used to determine the distribution and density of multiple species across the world (e.g. Van Strien et al. 2013; Scott et al. 2014, 2018; Walter et al. 2018; Okes and O’Riain 2019). We assessed the current distribution and apparent prevalence of mange infection in red foxes in urban areas of Britain using photographic and video images submitted by citizens via a web-based interface as recruited via a national televised program (see Scott et al. 2014). Our objectives were as follows: i) to update estimates of mange distribution and apparent overall prevalence in urban foxes in Britain; ii) to determine whether apparent prevalence currently varies geographically across urban Britain in the form of spatial clusters of high infection risk; or with distance away from a known source epizootic (i.e. Bristol) or other urban populations with mange; and iii) to explore whether prevalence is correlated with urban landscape features putatively affecting fox dispersal and density including city size, length of major roads, proportional public greenspace area, proportional rural area at the urban perimeter, and degree of perimeter habitat fragmentation. We predicted that mange would be widespread, and that frequency-dependent transmission would prevent extreme spatial fluctuations. However, we expected clusters of high risk to occur in the Southwest, North and East of England and in conurbations that are most closely grouped together, reflecting historical epizooty and urban fox distribution expansion. We also hypothesized that, if transmission were predominantly density-dependent, conurbations with extensive landscape features harboring lower densities of foxes (e.g. public greenspace (Scott et al. 2014)) would be associated with lower prevalence, as would highly fragmented environments, the latter as a consequence of restricted fox movements.
Methods
Study area
The target area comprised all conurbations in Britain i.e. towns and cities within England, Scotland and Wales. A conurbation was defined as an urban area with a human population of at least 75,000 (England and Wales) or 50,000 (Scotland) corresponding to a total area of 14,546 km2 (Office for National Statistics [ONS] 2004).
Photographic image collection
Photographic images were collected during April and May 2012, as part of a citizen science recruitment initiative to collate urban red fox sightings records for assessing distribution. Members of the UK public were asked to (anonymously) upload photographs or videos of any foxes photographed between 1st January 2012 and one week after the final broadcast date, together with the date, time and location, to a designated website. The website was produced in association with a nationwide series of television programs called “Foxes live: wild in the city” on Channel 4, which is one of the major publicly accessible terrestrial broadcasters in the U.K. Mange was not mentioned in the request for images, to reduce bias. An interactive link with Google maps enabled accurate georeferencing. Any images/videos with obviously erroneous locations (e.g. offshore) were removed prior to analysis and submitted images were screened for quality (see Scott et al. (2014) for full details of the data collection process). The project was given approval from The School of Pharmacy and Biomolecular Sciences Ethics committee, University of Brighton (Code: 1138). All duplicates were removed and images were excluded if <10% of fox body area was visible, resolution was poor, or the subject was too far away to assess condition.
Assessment of apparent mange prevalence
Mange prevalence was determined as ‘apparent prevalence’ following Carricondo-Sanchez et al. (2017) i.e. proportion of all foxes in images/videos with visible lesions and/or alopecia (considered to be infected) as a proportion of total number of foxes. Alopecia and skin lesions are predominantly visible on the rear of the animal and progress to the face (Baker et al. 2000; Newman et al. 2002; Kido et al. 2013). Mange was therefore determined from visible signs of extensive alopecia, raw skin, scabbing, lesions and hyperkeratosis in these specific areas. Seasonal moult, which is also characteristically visible at the rear, can be confused with mange. To distinguish between moult and mange-related alopecia, visibility of black guard hairs, which are only retained during moult (Maurel et al. 1986), were considered in the assessment. Assessment was conducted for every fox individual in the screened images/videos. Where images had multiple individuals, all individuals were assessed separately. Figure 1 shows examples of photos of varying quality, showing moult and mange. Following the assessment process, each image was assigned a score of 1,0 (mange present/absent). Where possible, sex was determined from visible sexual characteristics and head shape, age class from size and body proportions (Harris and Baker 2006). All images of juveniles (<3 months old) were consequently removed from the dataset for consistency, due to the progressive nature of mange resulting in greater visibility in adults (Newman et al. 2002; Walton and Currie 2007). A single assessor evaluated all images (NC). Verification was then undertaken by two additional assessors (AT and DS), each independently scoring 20 random individuals, and scores between the three assessors were compared.
Examples of submitted images of foxes showing a) good quality image, no mange; b) good quality image with mange (evidence of alopecia and hyperkeratosis on rump); c) poorer quality image with two foxes without mange, right side fox showing evidence of moult on left rear flank; d) poorer quality image with mange (evidence of alopecia and hyperkeratosis on rump, tail and face (copyright for research given when anonymously submitted))
Identification of spatial clusters of mange using scan analysis
The scan analysis software SATSCAN (www.satscan.org) Version 9.4.2 was used to identify potential spatial clusters of sarcoptic mange in urban foxes based on the locations of positive occurrence of mange. SATSCAN accounted for the unequal sampling of different areas, which was an inevitable consequence of the citizen science method of recruiting records. The software superimposed circular ‘windows’ over the study area, mapped to a central point, with the size of the circle varying continuously from zero to a user-defined maximum as it moved across the study area. This created a permutation model, where candidate clusters were the circles and the most likely true clusters were identified using Maximum Likelihood, i.e. clusters least likely to be due to chance, given the underlying sample of the population. Specifically, Maximum Likelihood ratios generated from the data were then compared with those derived from random clusters using Monte Carlo simulations. An initial two-tailed analysis was computed to identify both high risk and low risk clusters of apparent mange, with the alternative hypotheses based on lower or higher risk within each circle as compared to the outside. We extracted significant high-risk clusters at the 0.95 level, using a maximum cluster radius of 100 km, based on an observed urban fox dispersal distance of 70 km (Tolhurst et al. 2016) and a 90 km maximum dispersal distance estimated by Atterby et al. (2015).
Analysis of landscape predictors of apparent mange prevalence
GIS methods
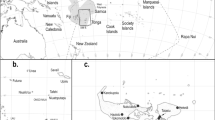
We analysed fox and landscape data from 11 conurbations: London, Bristol, Brighton, Bournemouth, South Hampshire, West Midlands, Liverpool, West Yorkshire, Greater Manchester, Edinburgh and Glasgow (Fig. 2 a and b). The cities/urban areas were selected using the criterion of at least 20 images being available for each, which we considered a viable threshold in terms of statistical validity, based on the data. The analysis was carried out in ArcGIS 10.3.1 with open source spatial data (Table 1). A spatial join of the fox and urban boundary datasets were used to select cities with >20 fox records which were then used as “focal cities” for analyses. For each city we generated a 40km2 “focal grid” using a 20 km square buffer from each city’s centroid and used this as our study boundary. This size was chosen to enable comparison between cities of different sizes, sample the fox population across the city based on urban fox home range sizes (Tolhurst et al. 2016) and provide a sufficient minimum sample size of images per city (n = 28 to 382). This buffer size created a grid that encompassed the entire city for each conurbation, whilst also allowing inclusion of bordering rural habitat and therefore peri-urban areas at the interface between urban and rural. This is important because peri-urban areas were found to be mange hotspots in a previous study (Carricondo-Sanchez et al. 2017). For each focal grid we calculated the percentage of images scoring positive for mange and used spatial overlay to derive explanatory landscape variables selected a priori to reflect our hypotheses. These variables included: absolute area of the focal city (Focal city area); proportion of the focal city area comprising combined public greenspace (Proportional greenspace area); proportion of the focal grid comprising rural habitat (Proportional rural area), total length of major roads (motorway and A-road combined) in the focal grid; a derived index of perimeter shape complexity at the urban/rural interface called the fractal dimension, which we used as a measure of habitat fragmentation; Euclidean distance to the nearest city with fox mange presence based on our survey (Distance to nearest city) and Euclidean distance to the origin of the most recent large-scale urban mange epizootic in Bristol (Distance to Bristol). Due to its large size and complexity, and the large number of associated images, separate analysis was additionally computed for London only. In this analysis, a 1 km square grid was overlaid to give 14 grid squares (Fig. 3) for which mange occurrence and landscape variables were extracted following an identical method.
Statistical methods
All analyses were computed in the R environment for statistical computing (Version 3.6.1). Putative landscape predictors of apparent mange prevalence were investigated using multiple linear regression models. We ran one analysis for each of two response variables: i) apparent prevalence in focal city for all cities including London (‘mangefc’); and ii) apparent prevalence in London only (‘mangeL’). Both responses were square root transformed to stabilise variances and thus assume a Gaussian error distribution. Each was then regressed against one or more of the explanatory landscape variables within a Generalised Linear Model (GLM) framework in the package lmer. For mangeL, a reduced set of explanatory variables were used, as appropriate for different areas of a single conurbation; these were Proportional greenspace area and Proportional rural area. We then generated separate models that contained all possible combinations of variables, excluding those which were nested or non-independent. Therefore, as the nearest city with mange could be Bristol, Distance to city and Distance to Bristol were not included in the same model. Likewise, Focal city area and Proportional rural area were not included together as the former was contained within the grid from which the latter was derived. The resulting models were then ranked based on weights derived from information criteria (Symonds and Moussalli 2011) where models associated with delta AICc (∆AICc) of less than 2 were substantially evidenced and those with AICc of between 2 and 4 were plausible (after Burnham and Anderson 2002). Model-averaged coefficients for the variables in the highest ranked models (∆AICc <2) were then generated. Model selection and averaging were computed using the R package MuMIn (Barton 2018).
Results
Apparent mange prevalence overall
Of the 2808 images submitted, 1521 were considered suitable for analysis (54%) and a total of 1901 individual foxes were assessed, i.e. where multiple foxes were present in images. Of these, 33% were juveniles and therefore excluded, leaving 1274 individuals in total, of which 6.5% were sub-adults and 60.5% adults. 192 images of foxes had visual symptoms of apparent mange (concurred by assessors in >95% of cases) representing 15.1% of the total.
Spatial clusters of mange
1074 spatial locations from 153 (of 201) counties were analysed using SATSCAN. These included the 192 records of mange. One-tailed analysis for high-risk clusters identified 11 clusters, only one of which was significant at the 95% level and encompassed an area that spanned parts of Northwest England (Liverpool and Greater Manchester) and the West Midlands (Fig. 4; Table 2). Apparent mange prevalence in this high-risk cluster was 37.1%, and therefore more than double prevalence overall. Our estimate of mange prevalence in Bristol was relatively high (20%) compared to other areas outside of the high-risk hotspot such as London, and Scottish cities, none of which exceeded 12% prevalence (Table 2).
Landscape predictors of apparent mange prevalence
The highest-ranked models for predicting apparent mange prevalence in focal cities contained the intercept only, Fractal dimension, and Distance to city (Table 3). Mange prevalence was positively associated with Fractal dimension and negatively associated with Distance to city (Table 4). For the London area only, no landscape variables were contained within the highest ranked models, i.e. the intercept only model was substantially better than all other models (Table 5).
Discussion
National distribution of mange prevalence
As predicted, mange was widespread in urban foxes in Great Britain and extreme spatial fluctuations in apparent prevalence were not detected. Mange spatial distribution was non-uniform, and the high-risk hotspot identified in Northwest and Central-west England is partially consistent with predictions based on recent shifts in urban fox distribution and historical epizooty (i.e. north and west of Bristol). However, there was no hotspot in the Southwest or East. Further, we found no association between apparent prevalence and distance to Bristol, although we did detect a relatively weak relationship where prevalence decreased with increasing distance from the nearest conurbation with mange. There has not been a largescale mange outbreak in Britain since the Bristol epizootic, which spread rapidly through urban populations nationwide within a few years (e.g. Simpson 2003). It is probable that the consequent absence of naïve populations, and correspondingly widespread enzootic infection, mean that present day prevalence is no more likely to be higher closer to original source epizootics than anywhere else. We therefore suggest that the weak effect of higher mange prevalence in cities that are closer to other cities (i.e. clustered conurbations) reflects increased fox movements (e.g. dispersal) between adjacent urban areas, rather than variable prevalence between conurbations. Increased dispersal may result from high rates of intra-specific competition where foxes occur at medium-to-high densities.
The urban landscape and mange
The lack of strong effects of multiple urban landscape features on apparent prevalence was surprising given the generally heterogenous nature of cities and associated variation in fox habitat quality. Some studies report broad-scale habitat effects on mange prevalence (e.g. higher at the urban periphery, Carricondo-Sanchez et al. 2017) although others show no pattern (e.g. Soulsbury et al. 2007). However, the current study is the first (to our knowledge) to specifically relate mange occurrence to habitat variation within cities rather than comparing essentially homogenous urban environments with wilderness or rural areas. Therefore, we might not expect our results to be consistent with other studies. In addition, in Britain urban foxes are known to positively select residential gardens (yards) where they are generally free from persecution and encounter essential resources including supplementary food, and den/layup sites e.g. under sheds or in mature vegetation (Saunders et al. 1993; Newman et al. 2003; Scott et al. 2018). Thus, if a difference existed between habitats within a city, we might expect it to be higher mange prevalence in conurbations with greater proportions of private greenspace (i.e. gardens/yards), relative to other areas e.g. public greenspace in which foxes occur at lower density (Scott et al. 2014). Even if transmission was predominantly frequency rather than density-dependent in our study system, the types of behaviours exhibited by resident foxes in private greenspace, such as cross-utilisation of lay-up sites, are likely to facilitate mange transfer (Soulsbury et al. 2007; Montecino-Latorre et al. 2019). Conversely, fox populations in residential gardens tend to be configured within stable territories with limited contact between neighbouring social groups (Baker et al. 2000), such that mange spread within the broader environment would be minimal. We were not able to test these theories, because the inclusion of private greenspace in addition to public greenspace in our GIS analysis would have created a mismatch at the spatial resolution required for meaningful interpretation of the photographic images. Nonetheless, further exploration of this issue is strongly recommended.
We interpret the higher mange prevalence values with increasing urban perimeter shape complexity in terms of increased fox movements at the urban-rural interface relative to the city interior. This contrasts with DeCandia et al. (2018) in Zurich, where limited dispersal occurred between sub-populations within a city. Our findings suggest that there are few barriers to dispersal either within or between UK cities, however further work is necessary to confirm this.
Mange effects and treatment
Mange can affect the feeding and movement behaviour of infected animals (Murray et al. 2015; Carricondo-Sanchez et al. 2017; Sűld et al. 2017) and infection is linked to poor body condition and nutritional status, which can alter individual position in dominance hierarchies (Soulsbury et al. 2007) and/or utilisation of habitats and resources (Carricondo-Sanchez et al. 2017). This may lead to an over-estimation of prevalence using our method if mangy foxes access anthropogenic resources more readily than healthy animals, or are less afraid of humans (e.g. Bornstein et al. 2001) and therefore urban residents are more likely to see and photograph them. Prevalence may also be affected by ad hoc treatment administered by rehabilitation centres or members of the public. The extent of such in situ medicating (usually with the parasiticide agent, ivermectin) and whether or how it affects mange prevalence and spread remain unknown. Mange treatment in wildlife is controversial, as there are potential side effects of ivermectin for target individuals and medication may also inadvertently enter the environment and food chain leading to drug resistance (Niedringhaus et al. 2019). Further research into this area is strongly recommended.
Limitations of the study
The method of image recruitment was necessarily non-random and further studies are recommended to locally validate the results. There may be bias away from photographing animals in poor condition as they are not aesthetically pleasing, hence underestimating the proportion with mange. One approach could collate spatial and temporal records of foxes with apparent mange form the RSPCA and wildlife centres across the UK. The study was conducted in the spring only, the initial focus being to determine the current distribution of the pre-breeding fox population. However, seasonal variation in mortality (Nimmervoll et al. 2013) and group size affecting intra-group contacts (Dorning and Harris 2017) mean that our results probably inadequately reflected prevalence across the annual cycle. Additionally, mange manifestation varies depending on mite burden, with ‘ordinary mange’ predominantly associated with hair loss, and ‘crusted mange’ presenting with severe hyperkeratosis and sero-cellular crusting (Carricondo-Sanchez et al. 2017). Hair loss is likely to be more apparent in spring due to moult and breeding alopecia (hair loss associated with pregnancy or postpartum period (Novak and Meyer 2009)) so there is a risk of misdiagnosis and consequent over-estimation of prevalence. Opposing bias may have resulted from the variation in symptoms with disease progression such that early-stage symptoms (e.g. small lesions) may have been overlooked. Further under-estimation may have arisen if signs were restricted to ‘invisible’ parts of the body such as the abdomen (i.e. ‘occult’ presentations; Alasaad et al. 2012). It is recommended that future studies compare visual and clinical assessment of mange by combining monitoring of captive animals of known disease status with remotely captured images. However, image selection and appraisal by the assessors was consistent, and all geographical areas were surveyed in the same season, hence we provided a relative measure of mange prevalence that was comparable across the dataset.
Conclusions
We show that citizen science can be an effective method for monitoring wildlife diseases for which symptoms are highly visible; in this case showing that hotspots of mange prevalence have shifted northward since a previous assessment, which partially reflects the expansion in fox distribution in recent decades. Our findings also suggest that fox movements such as dispersal are likely to be influential drivers of mange prevalence in British urban areas.
References
Alasaad S, Permunian R, Gakuya F, Mutinda M, Soriguer RC, Rossi L (2012) Sarcoptic-mange detector dogs used to identify infected animals during outbreaks in wildlife. BMC Vet Res 8:110. https://doi.org/10.1186/1746-6148-8-110
Arlian L, Morgan MS (2017) A review of Sarcoptes scabiei: past, present and future. Parasit Vectors 10(1). https://doi.org/10.1186/s13071-017-2234-1
Atterby H, Allnutt TR, MacNicoll AD, Jones EP, Smith GC (2015) Population genetic structure of the red fox (Vulpes vulpes) in the UK. Mamm Res 60:9–19. https://doi.org/10.1007/s13364-014-0209-6
Baker PJ, Harris S (2007) Urban mammals: what does the future hold? An analysis of the factors affecting patterns of use of residential gardens in Great Britain. Mammal Rev 37:297–315. https://doi.org/10.1111/j.1365-2907.2007.00102.x
Baker PJ, Funk SM, Harris S, White PCL (2000) Flexible spatial organization of urban foxes, Vulpes vulpes, before and during an outbreak of sarcoptic mange. Anim Behav 59:127–146. https://doi.org/10.1006/anbe.1999.1285
Barton K, (2018) Package ‘MuMIn’. R Environmental for Statistical Computing. Available at: https://cran.r-project.org/web/packages/MuMIn/MuMIn.pdf Last accessed 08/01/19
Bornstein S, Mörner T, Samuel WH (2001) Sarcoptes scabiei and sarcoptic mange. In: Samuel WM, Pybus MJ, Kocan A (eds) Parasitic diseases of wild mammals 2nd edition: 107–19. Iowa State University Press, Ames
Bornstein S, Frossling J, Naslund K, Zakrisson G, Mörner T (2006) Evaluation of a serological test (indirect ELISA) for the diagnosis of sarcoptic mange in red foxes (Vulpes vulpes). Vet Derma 17:411–416. https://doi.org/10.1111/j.1365-3164.2006.00548.x
Brochier B, De Blander H, Hanosset R, Berkvens D, Losson B, Saegerman C (2007) Echinococcus multilocularis and Toxocara canis in urban red foxes (Vulpes vulpes) in Brussels, Belgium. Prev Vet Med 80:65–73. https://doi.org/10.1016/j.prevetmed.2007.01.004
Burnham K, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York, p 488
Carricondo-Sanchez D, Odden M, Linnell JDC, Odden J (2017) The range of the mange: spatiotemporal patterns of sarcoptic mange in red foxes (Vulpes vulpes) as revealed by camera trapping. PLoS ONE 12(4):e0176200. https://doi.org/10.1371/journal.pone.0176200
Cypher BW, Rudd JL, Westall TL, Woods LW, Stephenson S, Foley JE, Richardson D, Clifford DL (2017) Sarcoptic mange in endangered kit foxes (Vulpes macrotis mutica): case histories, diagnoses and implications for conservation. J Wildl Dis 53:46–53. https://doi.org/10.7589/2016-05-098
Daszak P, Cunningham AA, Hyatt AD (2000) Emerging infectious diseases of wildlife - threats to biodiversity and human health. Science 287(5459):1756–1756. https://doi.org/10.1126/science.287.5452.443
Davidson RK, Bornstein S, Handeland K (2008) Long-term study of Sarcoptes scabiei infection in Norwegian red foxes (Vulpes vulpes) indicating host/parasite adaptation. Vet Parasitol 156(3-4):277–283. https://doi.org/10.1016/j.vetpar.2008.05.019
DeCandia AL, Brzeki KE, Heppenheimer E, Caro CV, Camenisch G, Wandeler P, Driscoll C, vonHoldt BM (2018) Urban colonization through multiple genetic lenses: the city-fox phenomenon revisited. Ecol Evol 9(4):2046–2060. https://doi.org/10.1002/ece3.4898
Devenish-Nelson ES, Richards SA, Harris S, Soulsbury C, Stevens PA (2014) Demonstrating frequency-dependent transmission of sarcoptic mange in red foxes. Biol Lett 10:20140524. https://doi.org/10.1098/rsbl.2014.0524
Dorning J, Harris S (2017) Dominance, gender, and season influence food patch use in a group-living, solitary foraging canid. Behav Ecol 28(5):1302–1313. https://doi.org/10.1093/beheco/arx092
Fuchs B, Zimmermann B, Wabakken P, Bornstein S, Mansson J, Evans AL, Liberg O, Sand H, Kindberg J, Agren EO, Arnemo JM (2016) Sarcoptic mange in the Scandinavian wolf Canis lupus population. BMC Vet Res 12:1–12. https://doi.org/10.1186/s12917-016-0780-y
Gakuya F, Ombui J, Maingi N, Muchemi G, Ogara W, Soriguer RC, Alasaad S (2012) Sarcoptic mange and cheetah conservation in Masai Mara (Kenya): epidemiological study in a wildlife/livestock system. Parasitology 139(12):1587–1595. https://doi.org/10.1017/S0031182012000935
Gehrt S, Riley SPD, Cypher BL (2010) Urban Carnivores: Ecology, Conflict and Conservation. John Hopkins University Press, Baltimore, p 283
Gortazar C, Villafuerte R, Blanco JC, De Luco DF (1998) Enzootic sarcoptic mange in red foxes in Spain. Zeitschrift fur Jagwissenschaft 44:251–256. https://doi.org/10.1007/BF02242030
Haas C, Origgi FC, Rossi S, López-Olvera JR, Rossi L, Castillo-Contreras R, Malmsten A, Dalin AM, Orusa R, Robetto S, Pignata L, Lavín S, Ryser-Degiorgis MP (2018) Serological survey in wild boar (Sus scrofa) in Switzerland and other European countries: Sarcoptes scabiei may be more widely distributed than previously thought. BMC Vet Res 14(117):1–10. https://doi.org/10.1186/s12917-018-1430-3
Harris S, Baker P (2006) Urban foxes (British natural history). Whittet Books, 152 pp
Kauhala K, Talvitie K, Vuorisalo T (2016) Encounters between medium-sized carnivores and humans in the city of Turku, SW Finland, with special reference to the red fox. Mamm Res 61:25–33. https://doi.org/10.1007/s13364-015-0250-0
Kido N, Itabashi M, Takahashi M, Futami M (2013) Epidemiology of sarcoptic mange in free-ranging raccoon dogs (Nyctereutes procyonides) in Yokohama, Japan. Vet Parasitol 191(1–2):102–107. https://doi.org/10.1016/j.vetpar.2012.07.026
Lindström ER, Morner T (1985) The spreading of sarcoptic mange among Swedish red foxes (Vulpes Vulpes) in relation to fox population dynamics. Rev Ecol (Terre Vie) 40:211–216
Lindström ER, Andrén H, Angelstam P, Cederlund G, Hörnfeldt B, Jäderberg L, Lemnell PA, Martinsson B, Sköld K, Swenson JE (1994) Disease reveals the predator: Sarcoptic mange, red fox predation, and prey populations. Ecology 75:1042–1049. https://doi.org/10.2307/1939428
Maurel D, Coutant C, Boissin-Agasse L, Boissin J (1986) Seasonal moulting patterns in three fur bearing mammals: the European badger (Meles meles L.), the red fox (Vulpes vulpes L.), and the mink (Mustela vison). A morphological and histological study. Can J Zool 64(8):1757–1764. https://doi.org/10.1139/z86-265
McKinney ML (2008) Effects of urbanization on species richness: a review of plants and animals. Urban Ecol 11:161–176. https://doi.org/10.1007/s11252-007-0045-4
Menzano A, Rambozzi L, Rossi L (2004) Outbreak of scabies in human beings, acquired from chamois (Rupicapra rupicapra). Vet Rec 155(18):568. https://doi.org/10.1136/vr.155.18.568
Montecino-Latorre D, Cypher BL, Rudd JL, Clifford DL, Mazet JAK, Foley JE (2019) Assessing the role of dens in the spread, establishment, and persistence of sarcoptic mange in an endangered canid. Epidemics. https://doi.org/10.1016/j.epidem.2019.01.001
Morgan ER, Tomlinson A, Hunter S, Nichols T, Roberts E, Fox MT, Taylor MA (2008) Angiostrongylus vasorum and Eucoleus aerophilus in foxes (Vulpes vulpes) in Great Britain. Vet Parasitol 14:48–57. https://doi.org/10.1016/j.vetpar.2008.02.030
Murray M, Edwards MA, Abercrombie B, St Clair CC (2015) Poor health is associated with use of anthropogenic resources in an urban carnivore. Proc R Soc Lond B Biol Sci:282. https://doi.org/10.1098/rspb.2015.0009
Newman TJ, Baker PJ, Harris S (2002) Nutritional condition and survival of red foxes with sarcoptic mange. Can J Zool 80:154–161. https://doi.org/10.1139/z01-216
Newman TJ, Baker PJ, Simcock E, Saunders G, White PCL, Harris S (2003) Changes in red fox habitat preference and rest site fidelity following a disease-induced population decline. Acta Theriol 48:79–91. https://doi.org/10.1007/BF03194268
Niedringhaus KD, Brown JD, Sweeley KM, Yabsley MJ (2019) A review of sarcoptic mange in north American wildlife. Inter J Parasit: Parasites Wildlife 9:285–297. https://doi.org/10.1016/j.ijppaw.2019.06.003
Nimmervoll H, Hoby S, Robert N, Lommano E, Welle M, Ryser-Degiorgis MP (2013) Pathology of sarcoptic mange in red foxes (Vulpes vulpes): macroscopic and histologic characterization of three disease stages. J Wildl Dis 49:91–102. https://doi.org/10.7589/2010-11-316
Nouvellet P, Donnelly CA, De Nardi M, Rhodes CJ, De Benedictis P, Citterio C et al (2013) Rabies and canine distemper virus epidemics in the red fox population of northern Italy (2006–2010). PLoS One 8:e61588. https://doi.org/10.1371/journal.pone.0061588
Novak MA, Meyer JS (2009) Alopecia; possible causes and treatments, particularly in captive nonhuman primates. Comp Med 59(1):18–26
Office for National Statistics [ONS] (2004) Census 2001. Office for National Statistics. www.ons.gov.uk/census/2011census/2011censusdata/2001censusdata
Okes NC, O’Riain MJ (2019) Can opportunistic citizen sightings assist in the monitoring of an elusive, crepuscular mammal in an urban environment? Urban Ecosyst 22:483–492. https://doi.org/10.1007/s11252-019-0829-3
Pisano S, Ryser-Degiorgis M, Rossi L, Peano A, Keckeis K, Roosje P (2019) Sarcoptic mange of fox origin in multiple farm animals and scabies in humans, Switzerland. Emerg Infect Dis 25(6):1235–1238. https://doi.org/10.3201/eid2506.18189
Plumer L, Davison J, Saarma U (2014) Rapid urbanization of red foxes in Estonia: distribution, behaviour, attacks on domestic animals, and health-risks related to zoonotic diseases. PLoS One 9(12). https://doi.org/10.1371/journal.pone.0115124
Saunders G, White PCL, Harris S, Rayner JMV (1993) Urban foxes (Vulpes vulpes): food acquisition, time and energy budgeting of a generalised predator. Symp Zool Soc Lond 65:215–234
Scott DM, Berg MJ, Tolhurst BA, Chauvenet ALM, Smith GC, Neaves K, Lochhead J, Baker PJ (2014) Changes in the distribution of red foxes (Vulpes vulpes) in urban areas in Great Britain: findings and limitations of a media-driven nationwide survey. PLoS One 9. https://doi.org/10.1371/journal.pone.0099059
Scott DM, Baker R, Charman N, Karlsson H, Yarnell R, Mill A, Smith G, Tolhurst B (2018) A citizen science based survey method for estimating the density of urban carnivores. PLoS One 13(5). https://doi.org/10.1371/journal.pone.0197445
Simpson VR (2003) Sarcoptic mange in foxes. Vet Rec 152:215–216
Singer A, Smith GC (2012) Emergency rabies control in a community of high-density hosts. BMC Vet Res 8:1–15. https://doi.org/10.1186/1746-6148-8-79
Smith GC, Wilkinson D (2003) Modelling control of rabies outbreaks in red fox populations to evaluate culling, vaccination and vaccination combined with fertility control. J Wildl Dis 39(2):278–286. https://doi.org/10.7589/0090-3558-39.2.278
Smith GC, Gangadharan B, Taylor Z, Laurenson MK, Bradshaw H, Hide G, Hughes JM, Dinkel A, Romig T, Craig PS (2003) Prevalence of zoonotic important parasites in the red fox (Vulpes vulpes) in Great Britain. Vet Parasitol 118:133–142. https://doi.org/10.1016/j.vetpar.2003.09.017
Soulsbury CD, White PCL (2015) Human-wildlife interactions in urban areas: a review of conflicts, benefits and opportunities. Wildl Res 42:541–553. https://doi.org/10.1071/WR14229
Soulsbury CD, Iossa G, Baker PJ, Cole SC, Funk SM, Harris S (2007) The impact of sarcoptic mange Sarcoptes scabiei on the British fox Vulpes vulpes population. Mammal Rev 37(4):278–296. https://doi.org/10.1111/j.1365-2907.2007.00100.x
Sűld K, Tammeleht E, Valdmann H, Saarma U (2017) Severe impact of sarcoptic mange on the movements and space use for one of its most important vector species, the raccoon dog. Vet Parasitol 243:67–70. https://doi.org/10.1016/j.vetpar.2017.05.029
Symonds MRE, Moussalli A (2011) A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav Ecol Sociobiol 65:13–21. https://doi.org/10.1007/s00265-010-1037-6
Taylor CS, Garcia Gato R, Learmount J, Aziz NA, Montgomery C, Rose H, Coulthwaite CL, Mcgarry JW, Forman DW, Allen S, Wall R, Morgan ER (2015) Increased prevalence and geographic spread of the cardiopulmonary nematode Angiostrongylus vasorum in fox populations in Great Britain. Parasitology 142:1190–1195
Teacher AG, Thomas JA, Barnes I (2011) Modern and ancient red fox (Vulpes vulpes) in Europe show an unusual lack of geographical and temporal structuring, and differing responses within the carnivores to historical climatic change. BMC Evol Biol 11:214. https://doi.org/10.1186/1471-2148-11-214
Tolhurst BA, Grogan A, Hughes H, Scott DM (2016) Effects of temporary captivity on ranging behaviour in urban red foxes (Vulpes vulpes). Appl Anim Behav Sci 181:182–190. https://doi.org/10.1016/j.applanim.2016.05.004
Tolnai Z, Szélla Z, Sprochb A, Szeredib L, Srétera T (2014) Dirofilaria immitis: an emerging parasite in dogs, red foxes and golden jackals in Hungary. Vet Parasitol 203:339–342. https://doi.org/10.1016/j.vetpar.2014.04.004
Tompkins DM, Dobson AP, Arneberg P, Begon ME, Cattadori IM, Greenman JV, Heesterbeek JAP, Hudson PJ, Newborn D, Pugliese A, Rizzoli AP, Rosà R, Rosso F, Wilson K (2002) Parasites and host population dynamics. In: Hudson PJ, Rizzoli A, Grenfell BT, Heesterbeek H (eds) DobsonAP (eds) the ecology of wildlife diseases pp. Oxford University Press, Oxford, pp 45–62
Uraguchi K, Uemo M, Iijima H, Saitoh T (2014) Demographic analyses of a fox population suffering from sarcoptic mange. J Wildl Manag 78(8):1356–1371. https://doi.org/10.1002/jwmg.794
Van Strien AJ, Van Swaay CAM, Termaat T (2013) Opportunistic citizen science data of animal species produce reliable estimates of distribution trends if analysed with occupancy models. J Appl Ecol 50:1450–1458. https://doi.org/10.1111/1365-2664.12158
Walter T, Zink R, Laaha G, Zaller JG, Heigl F (2018) Fox sightings in a city are related to certain land-use classes and sociodemographics: results from a citizen science project. BMC Ecol 18:50–11. https://doi.org/10.1186/s12898-018-0207-7
Walton SF, Currie BJ (2007) Problems in diagnosing scabies, a global disease in human and animal populations. Clin Microbiol Rev 20(2):268–279. https://doi.org/10.1128/CMR.00042-06
White PCL, Saunders G, Harris S (1996) Spatio-temporal patterns of home range use by foxes (Vulpes vulpes) in urban environments. J Anim Ecol:121–125. https://doi.org/10.2307/5705
Acknowledgments
We are grateful to Channel 4 for their funding of platform to collect images and associated data and all the production staff at Windfall Films. We would like to thank Phil Baker for input into the initial study of collecting distribution data and Graham Smith for discussions on mange. Finally, we would like to thank all the people who took the time and effort to submit their fox sightings with photographs.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Scott, D.M., Baker, R., Tomlinson, A. et al. Spatial distribution of sarcoptic mange (Sarcoptes scabiei) in urban foxes (Vulpes vulpes) in Great Britain as determined by citizen science. Urban Ecosyst 23, 1127–1140 (2020). https://doi.org/10.1007/s11252-020-00985-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-020-00985-5