Abstract
Brucella poses a great threat to animal and human health. Vaccination is the most promising strategy in the effort to control Brucella abortus (B. abortus) infection, but the currently used live vaccines interfere with diagnostic tests and could potentially result in disease outbreak. Therefore, new subunit vaccines and combined immunization strategies are currently under investigation. In this study, immunogenicity and protection ability of a recombinant adenovirus and plasmid DNA vaccine co-expressing P39 and lumazine synthase proteins of B. abortus were evaluated based on the construction of the two molecular vaccines. Four immunization strategies (single adenovirus, single DNA, adenovirus/DNA, DNA/adenovirus) were investigated. The results showed that the immunization strategy of DNA priming followed by adenovirus boosting induced robust humoral and cellular immune responses, and it significantly reduced the numbers of B. abortus in a mouse model. These results suggest that it could be a potential antigen candidate for development of a new subunit vaccine against B. abortus infection.
Similar content being viewed by others
Introduction
Brucellosis, caused by Brucella spp., is a highly infectious disease occurring in humans and various domestic and wild animals worldwide (Seleem et al. 2010; Walker and Blackburn 2015). This infection has wide-reaching economic effects and is a significant threat to global human health (Lopes et al. 2010; Godfroid et al. 2014; Vollmar et al. 2016). Although vaccination is among the most economic strategies, the current commercially available live vaccines can impede the detection of anti-Brucella antibodies conferred by wild strains (He and Xiang 2010; Christopher et al. 2010; Li et al. 2017). Thus, it is imperative to develop new diagnostic and preventative measures for the treatment of this disease.
Both DNA and live adenoviral vaccines offer an effect route for the activation of T helper (Th)1 and Th2 responses, and many studies have been reported on this work (Arévalo et al. 2009; Gabitzsch et al. 2011; Zhang and Zhou 2016; Tan et al. 2017). Presently, recombinant adenovirus expressing protective proteins of Brucella has not been reported, but some DNA vaccines related with Brucella have been studied (Luo et al. 2006; Jain et al. 2014; Golshani et al. 2015a). However, the results from these studies have demonstrated that single DNA vaccine confers a reduced antibody response and results in lower protection efficacy than the attenuated Brucella vaccines. Some strategies designed to improve the immune responses and protection efficacy of the DNA vaccines, including a combination of a DNA priming step and the homologous protein boosting, as well as co-expressing two proteins and the addition of adjuvant have been explored by others (Golshani et al. 2015a, b, 2016).
The P39 and lumazine synthase (LS) proteins are immunodominant and protective antigens conserved in animal Brucella abortus (B. abortus) (Luo et al. 2006; Tadepalli et al. 2016). The two proteins can elicit both humoral and cellular immune responses and are good candidates for use in future studies of vaccination against B. abortus.
In this study, the P39 and LS genes were cloned into adenovirus serotype 5 vector, and co-expressed. Additionally a plasmid carrying both genes was also constructed using pcDNA3.1 vector. The immunogenicity and protection ability conferred by four immunization strategies (single adenovirus, single DNA, adenovirus/DNA and DNA/adenovirus) were evaluated. The results offer a new avenue for vaccine development.
Materials and methods
Mice and Brucella
Specific pathogen-free grade female BALB/c mice aged 6–8 weeks old were purchased from the Center of Experimental Animals, Lanzhou Institute of Biological Products (Lanzhou, China). B. abortus strain CVCC12 (Biovar II) was obtained from China Veterinary Culture Collection Center (CVCC) (Beijing, China) and proliferated as instructed.
Ad-P39/LS and pcDNA-P39/LS vaccines
Recombinant adenovirus Ad-P39/LS co-expressing P39 and LS genes was propagated in HEK 293AD cells. Briefly, the P39 and LS genes were inserted into the multiple cloning site I (MCS I) and MCS II of the pQCXIX retroviral vector, respectively. These cloning events generated the pQC-LL/BP, in which P39-IRES-LS fragment was cloned into transfer vector pShuttle-CMV. The resulting positive plasmid pShuttle-P39/LS was linearized with PmeI and transformed into Escherichia coli BJ5183 competent cells carrying pAdEasy-1 skeleton vector to obtain recombinant adenovirus pAd-P39/LS. The pAd-P39/LS was cleaved with Pac I to expose its inverted terminal repeats and transfected into HEK 293AD cells. Finally, Ad-P39/LS were generated and propagated in these cells.
The P39-IRES-LS fragment was cloned into pcDNA3.1 vector with Not I and XhoI to obtain plasmid pcDNA-P39/LS, and the plasmids were transfected into HEK 293AD cells and propagated.
All the above works were previously accomplished (not published), and the relevant results were not displayed in this study.
Immunization and sera collection
Six test groups of mice (10 per group) were immunized by bilateral intramuscular injection into the gastrocnemius and boosted two times with the same dose with a 2-week interval, except for the mice of group 5 which were immunized only once with attenuated vaccine A19 strain. The detailed immunization strategies are shown in Table 1.
Serum samples were collected from all mice of the six groups before immunization, and at 14 days after the first and second immunizations, respectively. Sera was stored at −20 °C until they were analyzed for specific antibodies. Pre-immune serum samples were used as negative controls.
Detection of antibodies
Analysis of antigen-specific IgG, IgG1, and IgG2a antibodies in serum samples were performed using indirect enzyme-linked immune-sorbent assay (ELISA) as previously described (Golshani et al. 2015a, b). Briefly, 96-well microtiter plates (Costar, Bethesda, MD, USA) were coated with 10 μg/ml ultrasonicated B. abortus A19 strain overnight in carbonate buffer (pH 9.6) at 4 °C. The plates were blocked with 1% BSA in PBS for 30 min at 37 °C. After thorough washing with PBST, the serum samples were added to the plate and allowed to incubate for 30 min at 37 °C. The plates were washed again and were reacted with HRP-labeled anti-mouse IgG, IgG1, and IgG2a diluted in PBST at 1:1000 for 30 min at 37 °C. Plates were washed and developed with TMB, while being kept in a dark place for 10 min. Finally, stop solution was added, and optical density (OD) values were immediately measured at 450 nm using an ELISA reader. All samples were run in triplicate. The cutoff value for the assay was calculated as the mean specific OD plus 3 standard deviation (SD) for 30 pre-immunization serum samples.
Lymphoproliferation assay
The procedure was performed as in a previous report (Golshani et al. 2016). Two weeks after the second immunization, mice spleens were removed (five mice/group) and ground under sterile conditions using a 5-ml syringe plunger, and single-cell suspensions were obtained by filtration through stainless steel mesh. Splenocytes were isolated by mouse lymphoprep (Dakewe, Shenzhen, China) and placed into 96-well-plate with 100 μl/well at a density of 5×106 cells/ml in complete medium (RPMI 1640+10% FBS+100 U/ml penicillin/streptomycin). Cells were incubated with 5 μg/ml B. abortus A19 strain (10 μg/ml) or concanavalin A (5 μg/ml) or medium alone (negative control) in a 5% CO2 humidified incubator at 37 °C. The proliferative activity was measured using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, 5 mg/ml, Sigma) dye assay. The stimulation index (SI) was calculated as the ratio of the average OD570 of antigen-stimulated cells to the average OD570 of unstimulated cells.
Flow cytometric analysis of surface markers of lymphocytes
For flow assays, 2 ml of the splenocyte suspension (5×106 cells/ml) was centrifuged for 5 min at 2000 rpm, and the supernatants were discarded. The cells were washed one time with 1 ml fluorescence solution, and the supernatant was discarded. Then, the cells were suspended with 30 μl fluorescence solution. For each tube, 1 μl PerCP-CD3e, 1 μl PE-CD8a, and 0.5 μl FITC-CD4+ were added and mixed except for the control tube. The mixtures were incubated for 45 min in the dark and were then washed three times in the fluorescence solution. Finally, the cells were suspended in 200 μl fluorescence solution and filtered into a flow cytometry tube through a nylon membrane. The cells were then analyzed using a flow cytometer.
Cytokine assay
Splenocyte suspensions (5×106 cells/ml) were placed into a 24-well plate with 2 ml/well in duplicate. The fractions of A19 strain (1×108 cells/ml) treated with ultrasonication were placed into the plate at 10 μl/well. The plate was incubated for 120 h at 37 °C with 5% CO2. The supernatant of each well was collected for detection of IL-10 and IL-12.
Protection experiment
The experiment was performed in a BSL-3 laboratory as recommended (Golshani et al. 2016). Fourteen days after the second vaccination, five mice of each group were challenged by intraperitoneal route with 1×105 colony-forming unit (CFU) of B. abortus strain CVCC12 in 100 μl of PBS. At 4 weeks, post-challenge, spleens of the mice were aseptically removed and weighed. Each spleen was homogenized in PBS with 1:10 (g/ml, w/v) and serially diluted tenfold. Each dilution was applied to Brucella agar to determine the CFU. The results are presented using the mean±SD of Log10CFU per group. Log units of protection were obtained by subtracting the mean Log10CFU of the vaccinated group from the mean Log10CFU of the PBS control group. Log units of protection should increase following reduction of the bacteria.
Statistical analysis
One-way analysis of variance was carried out to analyze the differences between the groups using SPSS 20.0. Statistical significance was assumed at the p<0.05 level.
Results
Detection of antibodies
For group 1, the levels of IgG, IgG1, and IgG2a antibodies did not change during the whole process (p>0.05). For group 6, the three antibodies rapidly rose after the first immunization and kept high levels during the process.
The levels of IgG in the mice from groups 2 to 5 rose after the first immunization and significantly increased again after the second immunization. The IgG levels in group 5 was the highest, taking turn groups 4, 3 and 2. The OD450 values based on the specific IgG in all groups were shown in Fig. 1a.
For groups 2 to 5, the levels of IgG1 and IgG2a antibodies after the first immunization had no significant differences when compared with those before immunization (p>0.05). The two antibodies significantly rose after the second immunization. IgG2a antibodies were significantly higher compared with IgG1 in each corresponding group (p<0.05). OD450 values based specific IgG1 and IgG2a in all groups are shown in Fig. 1b, c, respectively.
Lymphoproliferation assay
Both strain A19 and ConA promote the proliferation of splenocyte T cells derived from the mice in groups 2 to 6, but no proliferation was observed in group 1. The SIs based-ConA were slightly higher than the values based-A19 in each corresponding group. The SIs from group 6 were the highest, followed by groups 5, 4, 3, and 2. The values from groups 5 and 4 were different from those in groups 3 and 2 (p<0.05). No significant differences were observed between groups 5 and 4 and groups 3 and 2. (p>0.05). These results are shown in Fig. 2.
Flow cytometric analysis of surface markers of lymphocytes
The percentages of CD3+ and CD4+ T cells from groups 2 to 6 were significantly high compared with those from group 1 (p<0.05). Although the percentages in group 6 were the highest, no significant differences were observed between groups 5 and 6 (p>0.05). The percentages had no differences between groups 2 and 3 (p>0.05). The percentages from group 4 were slightly high than those from groups 2 and 3. For CD8+, no statistically significant differences were obtained in groups 1 to 5 (p>0.05), but the value in group 6 was significantly higher compared with those from the other five groups (p<0.05). These results are displayed in Fig. 3.
Cytokine assay
No statistically significant differences in IL-10 were observed between groups 1 to 5 (p>0.05), but the level in group 6 was significantly higher in comparison with those from the other five groups (p<0.05). For IL-12, the levels in groups 2 to 6 were significantly higher than group 1. The value from group 6 was highest followed by groups 5 and 4 (p<0.05), with no difference in induced levels between groups 4 and 5, although they were elevated in these groups when compared with groups 2 and 3 which also had no major differences in induction of this cytokine (p>0.05). The values for this analysis are shown in Fig. 4.
Protection experiment
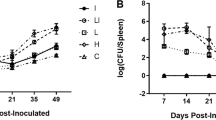
Protection was determined as a significant reduction in the number of bacteria in the spleens from immunized mice compared to the mice which received PBS. For group 5, the combined vaccines conferred significant protection with the log unit of 1.35, which was similar to that achieved by the live attenuated A19 vaccine. The immunization strategy in group 4 also revealed protection ability with a log unit of 1.16. The levels of protection were lower with a single vaccine approach as seen in groups 2 and 3. The detailed results are displayed in Table 2.
Discussion
Brucella is an intracellular pathogen, so it is very difficult to eradicate Brucella infection with antimicrobial agents. Vaccination is the most promising strategy to control the disease, but the currently used live vaccines interfere with diagnostic tests and have the potential risk of spreading the disease if the attenuation is incomplete (Christopher et al. 2010; Wang et al. 2015). In order to avoid the disadvantages, some new strategies including subunit recombinant protein vaccines (Du et al. 2016), vector vaccines based on E. coli (Gupta et al. 2012), Salmonella enterica (Zhao et al. 2009), Salmonella typhimurium (Kim et al. 2016), influenza viruses (Tabynov et al. 2014), and plasmid DNA vaccines (Luo et al. 2006; Cassataro et al. 2007; Jain et al. 2014) have all been evaluated for the prevention of Brucella infections. Presently, studies on recombinant adenovirus vaccines against some viruses and bacteria have been reported (Zhang and Zhou 2016; Tan et al. 2017), but the relevant research for Brucella has not been reported.
Moreover, some studies reveal that combined immunization with multivalent vaccines can elicit stronger immune responses and better protection against Brucella than the relevant univalent vaccines in mice model. Brucella Omp2b protein administered as Pro/Pro, DNA/DNA, or DNA/Pro regimen is capable of inducing vigorous humoral and cellular responses (Golshani et al. 2016). A recombinant fusion protein (rL7/L12-TOmp31) provided significant protection levels against Brucella melitensis (B. melitensis) and B. abortus challenges (Golshani et al. 2015a). The pcDNA-L7/L12-TOmp31 priming followed by rL7/L12-TOmp31 protein boosting led to improved protection against B. abortus or B. melitensis infection (Golshani et al. 2015b). A divalent genetic vaccine based on the L7/L12-Omp16 or L7/L12-P39 can elicit a stronger cellular immune response and better immunoprotection against B. abortus in comparison to single proteins (Luo et al. 2006). A 27-amino acid epitope derived from Omp31induced peptide- and BLS-specific Th1 and cytotoxic T responses (Cassataro et al. 2007).These above studies showed that divalent genetic vaccine encoding two proteins or agenetic vaccine priming and recombinant protein boosting could elicit stronger immune responses and provide protection against B. melitensis and B. abortus infection in BALB/c mice. Based on these works, we wanted to evaluate the immunogenicity and protection ability of recombinant adenovirus and plasmid DNA vaccines co-expressing P39 and LS proteins of B. abortus in BALB/c mice model. Furthermore, four immunization strategies (single adenovirus, single DNA, adenovirus/DNA, and DNA/adenovirus) were carried out. Since immunity against Brucella requires cell-mediated mechanisms (Th1 response), the antibody isotypes, splenocyte proliferative responses, T lymphocyte subsets, and the cytokines produced after immunization were evaluated.
In order to understand the humoral response, IgG levels of the mice were detected in this work. Th1 cells mainly mediate cellular immunity to accelerate IgG2a antibody (Carmi et al. 2015; Golshani et al. 2015b; Im et al. 2016), and IgG2a has the major role in immunity against Brucella by facilitating phagocytes. Therefore, both IgG1 and IgG2a isotypes were also done. After the second vaccination, IgG, IgG1, and IgG2a antibodies rapidly rose in all immunized mice except for the animals treated with PBS. The levels of IgG were the highest, followed by IgG2a and IgG1, and IgG2a exhibited dominance over IgG1. The pcDNA-P39/LS priming followed by Ad-P39/LS boosting (group 5) regimen induced higher antibody levels in comparison to single vaccines (groups 2 and 3) or Ad-P39/LS priming followed by pcDNA-P39/LS boosting strategy (group 4). The mice immunized with A19 (group 6) still exhibited the highest levels of antibody stimulation. These results indicated that pcDNA-P39/LS or Ad-P39/LS vaccines can elicit mixed Th1/Th2 type responses and Th1 response exhibited dominance.
In order to study cell-mediated immune response generated by pcDNA-P39/LS and Ad-P39/LS vaccines, splenocytes of mice from groups 1 to 6 were stimulated with A19 antigen or ConA. Based on the SIs, the splenocytes of all immunized mice showed significant proliferation than PBS control animals. The mice primed with pcDNA-P39/LS and boosted with Ad-P39/LS (group 5) were elicited better cellular immune response than those from other immunized mice, except the mice vaccinated with A19 antigen (group 6).
Since the cellular arm of the Th1 response is essential for conferring protection against Brucella infection (Jain et al. 2014), the splenocyte proliferative responses were evaluated. T cell subsets consisting of CD3+, CD4+, and CD8+ which produces Th1 or Th2 type cytokines were detected by FCM analysis. The results showed that CD3+ and CD4+ T cells from immunized mice rose significantly in comparison to PBS control animals. CD8+ cells had no changes among PBS control, pcDNA-P39/LS, Ad-P39/LS, Ad-P39/LS and pcDNA-P39/LS, and pcDNA-P39/LS and Ad-P39/L groups, but CD8+ cells from the mice (group 6) vaccinated with A19 had significant increase when compared with other groups. In addition, the percentages of CD3+ and CD4+T cells had no statistic differences between pcDNA-P39/LS and Ad-P39/L group and A19 group, although the values from A19 group were slightly higher than those from pcDNA-P39/LS and Ad-P39/L group. These results indicated that splenocytes from mice vaccinated with pcDNA-P39/LS or Ad-P39/LS vaccines were able to proliferate, and the two vaccines could induce mixed Th1/Th2-type responses.
To further understand the immune response, the cytokines IL-12 (Th1-type cytokine) and IL-10 (Th2-type cytokine) were tested, and Th1-type immune response in the form of high levels of IL-12 in all vaccinated groups was observed. Two step immunization strategies (groups 5 and 4) induced higher IL-12 levels in comparison to pcDNA-P39/LS or Ad-P39/LS regimen alone. The levels of IL-10 in all mice except for the animals in group 6 showed no significant changes. These results demonstrated that pcDNA-P39/LS priming followed by Ad-P39/LS boosting regimen could induce mainly Th1 type immune response.
Protection experiments were carried out to evaluate protective ability of the pcDNA-P39/LS or Ad-P39/LS vaccines. All the regimens using pcDNA-P39/LS or Ad-P39/LS vaccines could reduce the numbers of B. abortus CVCC12 strain in the spleens from the immunized mice. A combination of pcDNA-P39/LS priming with Ad-P39/LS boosting (group 5) significantly reduced the numbers of B. abortus CVCC12 strain when compared to the other three immunization strategies (groups 2, 3, and 4).
In conclusion, the current study demonstrated that pcDNA-P39/LS priming with Ad-P39/LS boosting regimen could effectively elicit robust humoral and cellular immune response and significantly reduce the numbers of B. abortus CVCC12 strain in BALB/c mice. This could be a potential antigen candidate following further studies in relevant target species.
References
Arévalo, M.T., Xu, Q., Paton, J.C., Hollingshead, S.K., Pichichero, M.E., Briles, D.E., Girgis, N., Zeng, M., 2009. Mucosal vaccination with a multicomponent adenovirus-vectored vaccine protects against Streptococcus pneumoniae infection in the lung. FEMS Immunology and Medical Microbiology, 55, 346–351.
Carmi, Y., Spitzer, M.H., Linde, I.L., Burt, B.M., Prestwood, T.R., Perlman, N., Davidson, M.G, Kenkel, J.A., Segal, E., Pusapati, G.V., Bhattacharya, N., Engleman, E.G., 2015.Allogeneic IgG combined with dendritic cell stimuli induce antitumour T-cell immunity. Nature, 521(7550), 99–104.
Cassataro, J., Velikovsky, C.A., Bruno, L., Estein, S.M., de la Barrera, S., Bowden, R., Fossati, C.A.,Giambartolomei, G.H. 2007. Improved immunogenicity of a vaccination regimen combining a DNA vaccine encoding Brucella melitensis outer membrane protein 31 (Omp31) and recombinant Omp31 boosting. Clinical and Vaccine Immunology, 14, 869–874.
Christopher, S., Umapathy, B.L., Ravikumar, K.L., 2010. Brucellosis: review on the recent trends in pathogenicity and laboratory diagnosis. Journal of Laboratory Physicians, 2(2), 55–60.
Du, Z.Q., Li, X., Wang, J.Y., 2016. Immunogenicity Analysis of a Novel Subunit Vaccine Candidate Molecule-Recombinant L7/L12 Ribosomal Protein of Brucella suis. Applied Biochemistry and Biotechnology, 179(8), 1445–1455.
Gabitzsch, E.S., Xu, Y., Balint, J.P. Jr., Balcaitis, S., Sanders-Beer, B., Jones, F.R., 2011. Induction and comparison of SIV immunity in Ad5 naïve and Ad5 immune non-human primates using an Ad5 [E1-, E2b-] based vaccine. Vaccine, 29, 8101–8107.
Godfroid, J., DeBolle, X., Roop, R.M, O'Callaghan, D., Tsolis, R.M, Baldwin, C., Santos, R.L., McGiven, J., Olsen, S., Nymo, I.H., Larsen, A., Al Dahouk, S., Letesson, J.J., 2014. The quest for a true One Health perspective of brucellosis. Revue Scientifique et Technique, 33(2), 521–538.
Golshani, M., Rafati, S., Gholami, E., Siadat, S. D., Oloomi, M., Bouzari, S., 2015a. Vaccination with recombinant L7/L12-Truncated Omp31 protein induces protection against Brucella infectionin BALB/c mice. Molecular Immunology, 65, 287–292.
Golshani, M., Rafati, S., Siadat, S. D., Nejati-Moheimani, M., Shahcheraghi, F., Arsang, A., Bouzari, S., 2015b. Improved immunogenicity and protective efficacy of a divalent DNA vaccine encoding Brucella L7/L12-truncated Omp31 fusion protein by a DNA priming and protein boosting regimen. Molecular Immunology, 66, 384–391.
Golshani, M., Rafati, S., Nejati-Moheimani, M., Ghasemian, M., Bouzari, S., 2016. Comparison of potential protection conferred by three immunization strategies (protein/protein, DNA/DNA, and DNA/protein) against Brucella infection using Omp2b in BALB/c Mice. Veterinary Microbiology, 197, 47–52.
Gupta, V.K., Radhakrishnan, G., Harms, J., Splitter, G., 2012. Invasive Escherichia coli vaccines expressing Brucella melitensis outer membrane proteins 31 or 16 or periplasmic protein BP26 confer protection in mice challenged with B. melitensis. Vaccine, 30(27), 4017–4022.
He, Y., Xiang, Z., 2010. Bioinformatics analysis of Brucella vaccines and vaccine targets using VIOLIN. Immunome Research, 6 (Suppl 1), S5.
Im, Y.B., Park, W.B., Jung, M., Kim, S., Yoo, H.S., 2016. Evaluation of Th1/Th2-related immune response against recombinant proteins of Brucella abortus infection in mice. Journal of Microbiology and Biotechnology, 26, 1132–1139.
Jain, S., Afley, P., Dohre, S.K., Saxena, N., Kumar, S., 2014. Evaluation of immunogenicity and protective efficacy of a plasmid DNA vaccine encoding ribosomal protein L9 of Brucella abortus in BALB/c mice. Vaccine, 32, 4537–4542.
Kim, W.K., Moon, J.Y., Kim, S., Hur, J., 2016. Comparison between immunization routes of live attenuated salmonella typhimurium strains expressing BCSP31, Omp3b, and SOD of Brucella abortus in Murine Model. Frontiers in Microbiology, 7, 550.
Li, M.T., Sun, G.Q., Zhang, W.Y., Jin, Z., 2017. Model-Based Evaluation of Strategies to Control Brucellosis in China.International Journal Environmental Research and Public Health, 14(3), 295.
Lopes, L.B., Nicolino, R., Haddad, J.P.A., 2010. Brucellosis – risk factors and prevalence: a review. Open Veterinary Science Journal, 4, 72–84.
Luo, D., Li, P., Xing, L., Zhao, G., Shi, W., Zhang, S., Wang, X., 2006. DNA vaccine encoding L7/L12-P39 of Brucella abortus induces protective immunity in BALB/c mice. Chinese Medical Journal, 119, 331–334.
Seleem, M.N., Boyle, S.M., Sriranganthan, N., 2010. Brucellosis. a re-emerging zoonosis. Veterinary Microbiology, 140, 392–398.
Tabynov, K., Sansyzbay, A., Kydyrbayev, Z., Yespembetov, B., Ryskeldinova, S., Zinina, N., Assanzhanova, N., Sultankulova, K., Sandybayev, N., Khairullin, B., Kuznetsova, I., Ferko, B., Egorov, A., 2014. Influenza viral vectors expressing the Brucella OMP16 or L7/L12 proteins as vaccines against B. abortus infection. Virology Journal, 11, 69.
Tadepalli, G., Singh, A.K., Balakrishna, K., Murali, H.S., Batra, H.V., 2016. Immunogenicity and protective efficacy of Brucella abortus recombinant protein cocktail (rOmp19+rP39) against B. abortus 544 and B. melltensis 16M in murine model. Molecular Immunology, 71, 34–41.
Tan, Q., Ma, S., Hu, J., Chen, X., Yu, Y., Tang, Z., Zang, G., 2017. Adenovirus vector harboring the HBcAg and tripeptidyl peptidase II genes induces potent cellular immune response in vivo. Cellular Physiology and Biochemistry, 41, 423–438.
Vollmar, P., Zange, S., Zöller, L., Erkel, J., Robert Thoma, B., 2016. Brucellosis, an overview and current aspects. Deutsche Medizinische Wochenschrift, 141(14), 1014–1018.
Walker, R., Blackburn, J., 2015. Biothreat Reduction and Economic Development: The Case of Animal Husbandry in Central Asia. Frontiers in Public Health, 3, 270.
Wang, W., Liao, Q., Wu, X., Hou, S., Wang, Y., Wu, J.,Shen, C., Chen, S., Allain, J.P., Li, C., 2015. Potential risk of blood transfusion-transmitted brucellosis in an endemic area of China.Transfusion, 55(3),586–592.
Zhang, C., Zhou, D., 2016. Adenoviral vector-based strategies against infectious disease and cancer. Human Vaccine & Immunotherapeutics,12(8), 2064–2074.
Zhao, Z., Li, M., Luo, D., Xing, L., Wu, S., Duan, Y.,Yang, P.,Wang X., 2009. Protection of mice from Brucella infection by immunization with attenuated Salmonella enterica serovar typhimurium expressing A L7/L12 and BLS fusion antigen of Brucella. Vaccine, 27, 5214–5219.
Acknowledgements
We sincerely thank Professor Changqing Qiu from Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences, for his valuable support. This work was financially supported by the Research Funds for Introduced Talents of Northwest University for Nationalities (xbmuyjrc2013) and by the Program for Changjiang Scholars and Innovative Research Team in University (Grant No. IRT13091).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study protocol was approved by the Animal Care and Use Committee of Life Science and Engineering College, Northwest University for Nationalities.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lin, GZ., Yang, JT., Wei, SC. et al. Immunogenicity of adenovirus and DNA vaccines co-expressing P39 and lumazine synthase proteins of Brucella abortus in BALB/c mice. Trop Anim Health Prod 50, 957–963 (2018). https://doi.org/10.1007/s11250-018-1517-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-018-1517-7