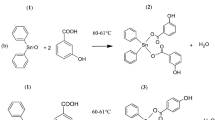
Abstract
Reaction of Na2[PdCl4] with two equivalents of 4,5-benzo-3H-1,2-dithiole-3-thione (btt) affords cis-[PdCl2(κ1-btt)2] (1), which provides a convenient entry into mixed-ligand btt complexes. Addition of one equivalent of a range of diamines or diphosphines gives the salts [Pd(κ1-btt)2(κ2-diamine)]Cl2 (2a–d) (diamine = en, dap, bipy, phen) and [Pd(btt)2(κ2-diphosphine)]Cl2 (3a–c) (diphosphine = dppe, dppp, dppf) in good yields. In contrast, two equivalents of dppm result in [Pd(κ1-btt)2(κ1-dppm)2]Cl2 (4), where the diphosphine binds in a monodentate fashion. Two equivalents of PPh3 result in a mixture of cis- and trans-isomers of [Pd(κ1-btt)2(PPh3)2]Cl2 (5a–b) (ca. 1:5 ratio); the pure trans-isomer 5b was isolated by ion-exchange chromatography. The cis-isomer 5a could be synthesized independently from the reaction of cis-[PdCl2(PPh3)2] with two equivalents of btt. In all of these complexes, the btt ligand binds in a monodentate manner through the exocyclic thione sulfur. The anti-tumor activities of representative examples, cis-[PdCl2(κ1-btt)2] (1), cis-[Pd(κ1-btt)2(κ1-dppm)2]Cl2 (4) and cis-[Pd(κ1-btt)2(PPh3)2] (5a), were evaluated by cell proliferation assays and phase-contrast microscopy against prostate cancer cell lines PC3, DU145 and LNCaP, with complexes 1 and 4 showing potent anti-proliferative effects (TGI values of 19.2 and 21.1 µg/mL, respectively) against LnCaP cells.
Graphical abstract









Similar content being viewed by others
References
Li KR, Yang SQ, Gong YQ, Yang H, Li XM, Zhao YX, Yao J, Jiang Q, Cao C (2016) Sci Rep 6:1–13
Jia Z, Misra BR, Zhu H, Li Y, Misra HP (2009) Neurotoxicology 30:1–9
Russell GK, Gupta RC, Vadhanam MV (2015) Mutat Res/Fundam Mol Mech Mutagen 774:25–32
Cuia YB, Mab SS, Zhang CY, Li BD, Yang B, Lv PJ, Xing Q, Huang T, Yange LG, Cao W, Guan FX (2018) Behav Brain Res 336:219–226
Kuo PC, Yu IC, Scofield BA, Brown DA, Curfman ET, Paraiso HC, Chang FL, Yen JH (2017) Brain Behave Immune 62:180–192
Kuo PC, Brown DA, Scofield BA, Paraiso HC, Wang PY, Yu IC, Yen JH (2018) Brain Behave Immune 336:219–226
Wallace JL, Caliendo G, Santagada V, Cirino G, Fiorucci S (2007) Gastroenterology 132:261–271
Munakata M, Kuroda-Sowa T, Maekawa M, Nakamura M, Akiyama S, Kitagawa S (1994) Inorg Chem 33:1284–1291
Munakata M, Dai J, Maekawa M, Kuroda-Sowa T, Fukui J (1994) J Chem Soc Chem Commun 2331–2332
Olk RM, Olk B, Dietzsch W, Kirmse R, Hoyer E (1991) Coord Chem Rev 117:99
Cassoux P, Valade L, Kobayashi H, Kobayashi A, Clark RA, Underhill AE (1991) Coord Chem Rev 110:115
Bryce MR (1991) Chem Soc Rev 20:355–390
Boukebbous K, Laifa EA, De Mallmann A (2016) IUCrData 1:x161688
Boukebbous K, Laifa EA, De Mallmann A, Taoufik M (2016) IUCrData 1:x161799
Laifa EA, Bendjeddou L, Boudraa N, Dahaoui S, Lecomte C (2009) Acta Cryst E 65:m1080–m1081
Raubenheimer HG, Kruger GJ, Marais CF (1984) Chem Soc Chem Commun 10:634–635
Dai J, Munakata M, Kuroda-Sowa T, Suenaga Y, Wu LP, Yamamoto M (1997) Inorg Chim Acta 255:163–166
Dai J, Munakata M, Wu LP, Kuroda-Sowa T, Suenaga Y (1997) Inorg Chim Acta 258:65–69
Drew MGB, Kisenyi JM, Parish RV (1987) J Chem Soc. DaltonTrans 7:1605–1609
Al-Jibori SA, Khaleel TF, Ahmed SA, Al-Hayaly LJ, Merzweiler K, Wagner C, Hogarth G (2012) Polyhedron 41:20–24
Al-Jibori SA, Al-Jibori QK, Schmidt H, Merzweiler K, Wagner C, Hogarth G (2013) Inorg Chim Acta 402:69–74
Al-Jibori SA, Al-Jibori MH, Hogarth G (2013) Inorg Chim Acta 398:117–123
Al-Jibori SA, Al-Jibori GH, Al-Hayaly LJ, Wagner C, Schmidt H, Timur S, Barlas FB, Subasi E, Ghosh S, Hogarth G (2014) J Inorg Biochem 14:55–57
Al-Jibori SA, Al-Bayati MMA, Gergees HM, Wagner C, Hogarth G (2017) Inorg Chim Acta 459:73–79
Saha M, Nasani R, Das M, Mobin SM, Pathak B, Mukhopadhyay S (2014) Inorg Chem Front 1:599–610
Nakamoto K (2009) Infrared and Raman spectra of inorganic and coordination compounds part B Applications in coordination, organometallic, and bioinorganic chemistry, 6th edn. Wiley, Hoboken
Gmeiner WH, Willingham MC, Bourland JD, Hatcher HC, Smith TL, Jr D’Agostino R B, Blackstock W (2014) J Clin Oncol Res 2:1028
Kawabata R, Oie S, Takahashi M, Kanayama H, Oka T, Itoh K (2011) Int J Oncol 38:1489–1500
Hasegawa M, Miyajima A, Kosaka T, Yasumizu Y, Tanaka N, Maeda T, Shirotake S, Ide H, Kikuchi E, Oya M (2012) Int J Cancer 130:431–442
Acknowledgements
We thank Tikrit University, Iraq, and Tokat Gaziosmanpaşa University, Turkey, for their supports.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-Jibori, S.A., Ulghafoor, M.A., Karadağ, A. et al. Synthesis, characterization and anti-tumor activity of Pd(II) complexes with 4,5-benzo-3H-1,2-dithiole-3-thione. Transit Met Chem 44, 575–583 (2019). https://doi.org/10.1007/s11243-019-00314-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-019-00314-6