Abstract
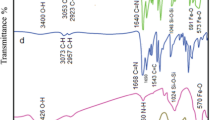
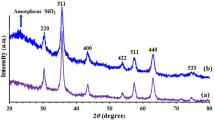
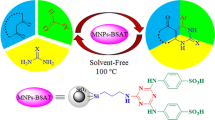
A magnetically recoverable nanocatalyst was synthesized by covalent binding of a Schiff base ligand, namely N,N′-bis(Salicylidene)-1,3-diaminopropane-2-ol (H2salpn), onto the surface of silica-coated magnetic CuFe2O4 nanoparticles, followed by complexation with MnCl2. The resulting core–shell nanoparticles were characterized by spectroscopic and microscopic methods, including FTIR, XRD, VSM, TGA elemental analysis, TEM, and SEM. The Mn content was determined by ICP analysis. The nanoparticles were investigated as a catalyst for the selective oxidation of alcohols to the corresponding carbonyl compounds with tertiary-butyl hydrogen peroxide. The catalyst can be magnetically separated for reuse, with no noticeable loss of activity in subsequent reaction cycles. FTIR, VSM, and leaching experiments after three successive cycles confirmed that the catalyst was strongly anchored to the magnetic nanoparticles. A suitable mechanism for the reaction is proposed.









Similar content being viewed by others
References
Shambayati S, Schreiber S, Trost B, Fleming I (1991) In: Trost BM (ed) Comprehensive organic synthesis. Pargamon Press, New York
Sheldon R (2012) Metal-catalyzed oxidations of organic compounds: mechanistic principles and synthetic methodology including biochemical processes. Elsevier, Amsterdam
Carey JS, Laffan D, Thomson C, Williams MT (2006) Analysis of the reactions used for the preparation of drug candidate molecules. Org Biomol Chem 4(12):2337–2347
Dugger RW, Ragan JA, Ripin DHB (2005) Survey of GMP bulk reactions run in a research facility between 1985 and 2002. Org Process Res Dev 9(3):253–258
Arends IW, Sheldon RA (2011) Modern oxidation of alcohols using environmentally benign oxidants. ChemInform 42(44)
Sheldon RA, Arends I, Dijksman A (2000) New developments in catalytic alcohol oxidations for fine chemicals synthesis. Catal Today 57(1):157–166
Sheldon RA, Arends IW, ten Brink G-J, Dijksman A (2002) Green, catalytic oxidations of alcohols. Acc Chem Res 35(9):774–781
Amato ME, Ballistreri FP, Pappalardo A, Tomaselli GA, Toscano RM, Williams DJ (2005) Novel chiral (Salen) MnIII complexes containing a Calix [4] arene unit as catalysts for enantioselective epoxidation reactions of (Z)-aryl alkenes. Eur J Org Chem 16:3562–3570
Moghadam M, Tangestaninejad S, Mirkhani V, Mohammadpoor-Baltork I, Mirjafari A, Mirbagheri NS (2010) Multi-wall carbon nanotubes supported molybdenum hexacarbonyl: an efficient and highly reusable catalyst for epoxidation of alkenes with tert-butyl hydroperoxide. J Mol Catal A Chem 329(1):44–49
Ulmann PA, Braunschweig AB, Lee O-S, Wiester MJ, Schatz GC, Mirkin CA (2009) Inversion of product selectivity in an enzyme-inspired metallosupramolecular tweezer catalyzed epoxidation reaction. Chem Commun 34:5121–5123
Wei S, Tang Y, Xu X, Xu G, Yu Y, Sun Y, Zheng Y (2011) A new heterogeneous chiral (salen) manganese(III) system for enantioselective epoxidation of non-functionalized olefins. Appl Organomet Chem 25(2):146–153
Yang JY, Liu SY, Korendovych IV, Rybak-Akimova EV, Nocera DG (2008) Hangman salen platforms containing dibenzofuran scaffolds. Chemsuschem 1(11):941–949
Yu K, Lou L-L, Ding F, Wang S, Wang Z, Liu S (2006) Highly enantioselective epoxidation of olefins by Mn(III) salen complex immobilized on MCM-48. Catal Commun 7(3):170–172
Kureshy RI, Ahmad I, Noor-ul HK, Abdi SH, Pathak K, Jasra RV (2005) Encapsulation of a chiral Mn III (salen) complex in ordered mesoporous silicas: an approach towards heterogenizing asymmetric epoxidation catalysts for non-functionalized alkenes. Tetrahedron Asymmetry 16(21):3562–3569
Kureshy RI, Ahmad I, Noor-ul HK, Abdi SH, Pathak K, Jasra RV (2006) Chiral Mn(III) salen complexes covalently bonded on modified MCM-41 and SBA-15 as efficient catalysts for enantioselective epoxidation of nonfunctionalized alkenes. J Catal 238(1):134–141
Ma L, Su F, Zhang X, Song D, Guo Y, Hu J (2014) Epoxidation of alkenes catalyzed by highly ordered mesoporous manganese–salen-based hybrid catalysts. Microporous Mesoporous Mater 184:37–46
Zhang H, Wang YM, Zhang L, Gerritsen G, Abbenhuis HC, van Santen RA, Li C (2008) Enantioselective epoxidation of β-methylstyrene catalyzed by immobilized Mn (salen) catalysts in different mesoporous silica supports. J Catal 256(2):226–236
Zhang H, Zhang Y, Li C (2006) Enantioselective epoxidation of unfunctionalized olefins catalyzed by the Mn (salen) catalysts immobilized in the nanopores of mesoporous materials. J Catal 238(2):369–381
Gong B, Fu X, Chen J, Li Y, Zou X, Tu X, Ding P, Ma L (2009) Synthesis of a new type of immobilized chiral salen Mn(III) complex as effective catalysts for asymmetric epoxidation of unfunctionalized olefins. J Catal 262(1):9–17
Tang X, Tang Y, Xu G, Wei S, Sun Y (2008) Highly enantioselective epoxidation of styrene and α-methylstyrene catalyzed by new doubly-immobilized chiral (salen) Mn(III) catalysts. Catal Commun 10(3):317–320
Silva AR, Freire C, de Castro B (2004) Jacobsen catalyst anchored onto an activated carbon as an enantioselective heterogeneous catalyst for the epoxidation of alkenes. Carbon 42(14):3027–3030
Tu X, Fu X, Hu X, Li Y (2010) Chiral salen Mn(III) immobilized on sulfoalkyl modified ZSP-IPPA as an effective catalyst for asymmetric epoxidation of unfunctionalized olefins. Inorg Chem Commun 13(3):404–407
Silva AR, Budarin V, Clark JH, Freire C, De Castro B (2007) Organo-functionalized activated carbons as supports for the covalent attachment of a chiral manganese(III) salen complex. Carbon 45(10):1951–1964
Chomoucka J, Drbohlavova J, Huska D, Adam V, Kizek R, Hubalek J (2010) Magnetic nanoparticles and targeted drug delivering. Pharmacol Res 62(2):144–149
Laurent S, Dutz S, Häfeli UO, Mahmoudi M (2011) Magnetic fluid hyperthermia: focus on superparamagnetic iron oxide nanoparticles. Adv Coll Interface Sci 166(1):8–23
Mahmoudi M, Sant S, Wang B, Laurent S, Sen T (2011) Superparamagnetic iron oxide nanoparticles (SPIONs): development, surface modification and applications in chemotherapy. Adv Drug Deliv Rev 63(1):24–46
Sun C, Lee JS, Zhang M (2008) Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Deliv Rev 60(11):1252–1265
Veiseh O, Gunn JW, Zhang M (2010) Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv Drug Deliv Rev 62(3):284–304
Zhao X, Bao XY, Guo W, Lee FY (2006) Immobilizing catalysts on porous materials. Mater Today 9(3):32–39
McCarthy JR, Weissleder R (2008) Multifunctional magnetic nanoparticles for targeted imaging and therapy. Adv Drug Deliv Rev 60(11):1241–1251
Shokouhimehr M, Piao Y, Kim J, Jang Y, Hyeon T (2007) A magnetically recyclable nanocomposite catalyst for olefin epoxidation. Angew Chem Int Ed 46(37):7039–7043
Saeednia S, Ardakani MH, Parizi ZP, Hafshejani MT (2016) Synthesis and characterization of a magnetically recoverable molybdenum(VI) nanocatalyst for eco-friendly oxidation of alcohols. Transit Met Chem 41(7):767–774
Garza-Navarro M, González-González V, Torres-Castro A, Hinojosa M, García-Loera A, José-Yacamán M (2010) Elaboration of superparamagnetic cobalt–ferrite nanocomposites from films of chitosan chelates. J Appl Polym Sci 117(2):785–792
Hu C, Gao Z, Yang X (2008) One-pot low temperature synthesis of MFe2O4 (M = Co, Ni, Zn) superparamagnetic nanocrystals. J Magn Magn Mater 320(8):L70–L73
Gadkari AB, Shinde TJ, Vasambekar PN (2011) Ferrite gas sensors. IEEE Sens J 11(4):849–861
Pershina A, Sazonov A, Ogorodova L (2010) Evaluation of the resistance of DNA immobilized on ferrimagnetic particles of cobalt ferrite nanopowder against nuclease cleavage. Bull Exp Biol Med 149(1):67–69
Esmaeilpour M, Sardarian AR, Javidi J (2012) Schiff base complex of metal ions supported on superparamagnetic Fe3O4@SiO2 nanoparticles: an efficient, selective and recyclable catalyst for synthesis of 1,1-diacetates from aldehydes under solvent-free conditions. Appl Catal A 445:359–367
Kapilevich L, D’yakova EY, Nosarev A, Zaitseva T, Petlina Z, Ogorodova L, Ageev B, Magaeva A, Itin V, Terekhova AO (2010) Effect of nanodisperse ferrite cobalt (CoFe2O4) particles on contractile reactions in guinea pigs airways. Bull Exp Biol Med 149(1):70–72
Yi DK, Lee SS, Ying JY (2006) Synthesis and applications of magnetic nanocomposite catalysts. Chem Mater 18(10):2459–2461
Yoon TJ, Yu KN, Kim E, Kim JS, Kim BG, Yun SH, Sohn BH, Cho MH, Lee JK, Park SB (2006) Specific targeting, cell sorting, and bioimaging with smart magnetic silica core–shell nanomaterials. Small 2(2):209–215
Ge Q, Huang Y, Qiu F, Li S (1998) Bifunctional catalysts for conversion of synthesis gas to dimethyl ether. Appl Catal A 167(1):23–30
Bagherzadeh M, Mortazavi-Manesh A (2015) Immobilized manganese porphyrin on functionalized magnetic nanoparticles via axial ligation: efficient and recyclable nanocatalyst for oxidation reactions. J Coord Chem 68(13):2347–2360
Esmaeilpour M, Javidi J, Dodeji FN, Abarghoui MM (2014) M (II) Schiff base complexes (M = zinc, manganese, cadmium, cobalt, copper, nickel, iron, and palladium) supported on superparamagnetic Fe3O4@SiO2 nanoparticles: synthesis, characterization and catalytic activity for Sonogashira-Hagihara coupling reactions. Transit Met Chem 39(7):797–809
Sun W, Wang H, Xia C, Li J, Zhao P (2003) Chiral-Mn (Salen)-complex-catalyzed kinetic resolution of secondary alcohols in water. Angew Chem Int Ed 42(9):1042–1044
Dandia A, Jain AK, Sharma S (2013) CuFe2O4 nanoparticles as a highly efficient and magnetically recoverable catalyst for the synthesis of medicinally privileged spiropyrimidine scaffolds. RSC Adv 3(9):2924–2934
Sobhani S, Pakdin-Parizi Z (2014) Lanthanum(III) triflate supported on nanomagnetic γ-Fe2O3: a new magnetically recyclable heterogeneous Lewis acid for the one-pot synthesis of β-phosphonomalonates. RSC Adv 4(25):13071–13077
Masteri-Farahani M, Tayyebi N (2011) A new magnetically recoverable nanocatalyst for epoxidation of olefins. J Mol Catal A Chem 348(1):83–87
Coleman W, Taylor L (1971) Pentadentate ligands. I. Nickel(II) complexes of the linear Schiff base ligands derived from substituted salicylaldehydes and diethylenetriamine and 2,2′-bis (aminopropyl) amine. Inorg Chem 10(10):2195–2199
Jia M, Thiel WR (2002) Oxodiperoxo molybdenum modified mesoporous MCM-41 materials for the catalytic epoxidation of cyclooctene. Chem Commun 20:2392–2393
Mukhopadhyay R, Bhattacharjee S, Bhattacharyya R (1994) Ligand control on the synthesis and redox potency of mononuclear manganese-(III) and-(IV) complexes with tridentate ONS co-ordination. J Chem Soc Dalton Trans 19:2799–2804
Panella B, Vargas A, Baiker A (2009) Magnetically separable Pt catalyst for asymmetric hydrogenation. J Catal 261(1):88–93
Mahdavi V, Mardani M (2015) Mn (Salen) Cl complexes immobilized on SBA-15 functionalized with amine as an efficient, selective and recyclable catalyst for benzyl alcohol oxidation by TBHP: the effects of Mn loading and reaction conditions. Res Chem Intermed 41(11):8907–8927
Oh NY, Suh Y, Park MJ, Seo MS, Kim J, Nam W (2005) Mechanistic insight into alcohol oxidation by high-valent iron–oxo complexes of heme and nonheme ligands. Angew Chem 117(27):4307–4311
Rahimi R, Ghoreishi SZ, Dekamin MG (2012) Immobilized metalloporphyrins on 3-aminopropyl-functionalized silica support as heterogeneous catalysts for selective oxidation of primary and secondary alcohols. Monatshefte für Chemie-Chem Mon 143(7):1031–1038
Mavis ME, Yolacan C, Aydogan F (2010) An investigation of the catalytic potential of mono-and dicationic imidazolium N-heterocyclic carbenes in the benzoin condensation. Tetrahedron Lett 51(34):4509–4511
Zhou Q, Wan Z, Yuan X, Luo J (2016) A new magnetic nanoparticle-supported Schiff base complex of manganese: an efficient and recyclable catalyst for selective oxidation of alcohols. Appl Organomet Chem 30(4):215–220
Nasseri MA, Mohammadinezhad A, Salimi M (2015) A cellulose-supported Mn (salen) Cl complex as an efficient heterogeneous catalyst for the selective oxidation of benzylic alcohols. J Iran Chem Soc 12(1):81–86
Mahdavi V, Mardani M (2012) Selective oxidation of benzyl alcohol with tert-butylhydroperoxide catalysed via Mn(II) 2,2-bipyridine complexes immobilized over the mesoporous hexagonal molecular sieves (HMS). J Chem Sci 124(5):1107–1115
Mahdavi V, Mardani M, Malekhosseini M (2008) Oxidation of alcohols with tert-butylhydroperoxide catalyzed by Mn(II) complexes immobilized in the pore channels of mesoporous hexagonal molecular sieves (HMS). Catal Commun 9(13):2201–2204
Acknowledgements
The support of this work by Vali-e-Asr University of Rafsanjan is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tavakoli Hafshejani, M., Saeednia, S., Hatefi Ardakani, M. et al. A manganese Schiff base complex immobilized on copper–ferrite magnetic nanoparticles as an efficient and recyclable nanocatalyst for selective oxidation of alcohols. Transit Met Chem 43, 579–589 (2018). https://doi.org/10.1007/s11243-018-0244-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-018-0244-2