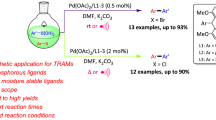
Abstract
A series of acetato-bridged [C^X]-type (C = aryl carbanion, X = N, P) palladacycles (1–5) of the general formula [Pd(μ-CH3COO)(C^X)]2 were synthesized as metal precursors via slightly modified procedures. However, in the case of complex 5 with Dpbp (Dpbp = 2′-(diphenylphosphino-κP)[1,1′-biphenyl]-2-yl-κC) as the supporting C^P ligand, an unexpected dinuclear complex [Pd(μ-CO2)(Dpbp)]2 (6) was obtained as a by-product and structurally determined by X-ray crystallography. The reactions of complexes 1–4 with 2-(diphenylphosphino)benzoic acid conveniently afforded four carboxylate-functionalized phosphine complexes [Pd(C^N)(Dpb)] (Dbp = 2-(diphenylphosphino-κP)benzoato-κO, 7–10), two of which (9/10) are newly synthesized in the present work and have been fully characterized. A comparative catalytic study revealed that complex [Pd(Ppy)(Dpb)] (7) (Ppy = 2-(2-pyridinyl-κN)phenyl-κC) is the best performer in Suzuki cross-couplings in H2O. In addition, complex 7 exhibits much better catalytic activity compared to the non-functionalized phosphine equivalent [Pd(OAc)(PPh3)(Ppy)] (11), which clearly indicates the superiority of incorporating a carboxylate-functionalized phosphine ligand into the palladacycles. A preliminary mechanistic study uncovered a different precatalyst initiation pathway compared to other known analogues of catalyst precursors.










Similar content being viewed by others
Notes
References
Dupont J, Pfeffer M (2008) Palladacycles: synthesis, characterization and applications. Wiley-VCH, Weinheim
Kapdi AR, Fairlamb IJS (2014) Chem Soc Rev 43:4751–4777
Dupont J, Consorti CS, Spencer J (2005) Chem Rev 105:2527–2571
Lyons TW, Sanford MS (2010) Chem Rev 110:1147–1169
Dunina VV, Gorunova ON (2004) Russ Chem Rev 73:309–350
Beletskaya IP, Cheprakov AV (2004) J Organomet Chem 689:4055–4082
Omae I (2004) Coord Chem Rev 248:995–1023
Bedford RB, Cazin CSJ, Holder D (2004) Coord Chem Rev 248:2283–2321
Bellina F, Carpita A, Rossi R (2004) Synthesis 15:2419–2440
Singleton JT (2003) Tetrahedron 59:1837–1857
Bedford RB (2003) Chem. Commun. 15:1787–1796
van der Boom ME, Milstein D (2003) Chem Rev 103:1759–1792
Albrecht M, van Koten G (2001) Angew Chem Int Ed 40:3750–3781
Dupont J, Pfeffer M, Spencer J (2001) Eur J Inorg Chem 8:1917–1927
Hartwig JF (2010) Organotransition metal chemistry, from bonding to catalysis. University Science Books, New York
Sharma AK, Joshi H, Bhaskar R, Kumar S, Singh AK (2017) Dalton Trans 46:2485–2496
Hu H, Qu F, Gerlach DL, Shaughnessy KH (2017) ACS Catal 7:2516–2527
Fath RH, Hoseini SJ (2017) J Organomet Chem 828:16–23
Iwasaki M, Nashihara Y (2016) Dalton Trans 45:15278–15284
Xiao ZQ, Xu C, Li HM, Han X, Wang ZQ, Fu WJ, Hao XQ, Song MP (2015) Transition Met Chem 40:501–508
Han X, Li HM, Xu C, Xiao ZQ, Wang ZQ, Fu WJ, Hao XQ, Song MP (2016) Transition Met Chem 41:403–411
Sánchez G, García J, Meseguer D, Serrano JL, García L, Pérez J, López G (2003) Dalton Trans 24:4709–4717
Sánchez G, García J, Meseguer D, Serrano JL, García L, Pérez J, López G (2004) Inorg Chim Acta 357:4568–4576
Sánchez G, Serrano JL, Moral MA, Pérez J, Molins E, López G (1999) Polyhedron 18:3057–3064
Sánchez G, García J, Liu M, García L, Pérez J, Pérez E, Serrano JL (2013) J Coord Chem 16:2919–2929
Rmírez-López P, Ros A, Romero-Arenas A, Iglesias-Sigüenza J, Fernández R, Lassaletta JM (2016) J Am Chem Soc 138:12053–12056
Aleock NW, Brown JM, Pearson M, Woodward S (1992) Tetrahedron Asymmetry 3:17–20
Seechurn CCCJ, Kitching MO, Colacot TJ, Snieckus V (2012) Angew Chem Int Ed 51:5062–5085
Nicolaou KC, Bulger PG, Sarlah D (2005) Angew Chem Int Ed 44:4442–4489
Fortman GC, Nolan SP (2011) Chem Soc Rev 40:5151–5169
Bedford RB, Cazin CJ (2001) Chem Commun 17:1540–1541
Bedford RB, Hazelwood SL, Horton PN, Hursthouse MB (2003) Dalton Trans 21:4164–4174
Bedford RB, Cazin CSJ, Hursthouse MB, Light ME, Scordia VJM (2004) Dalton Trans 22:3864–3868
Bedford RB, Cazin CSJ (2003) Organometallics 22:987–999
Bedford RB, Cazin CSJ, Hursthouse MB, Light ME, Pike KJ, Wimperis S (2001) J Organomet Chem 633:173–181
Chatterjee A, Ward TR (2016) Catal Lett 146:820–840
Mattiello S, Rooney M, Sanzone A, Brazzo P, Sassi M, Beverina L (2017) Org Lett 19:654–657
Zong Y, Wang J, He Y, Yue G, Wang X (2016) RSC Adv 6:89621–89626
Ghorbani-Choghamarani A, Tahmasbi B, Moradi P (2016) RSC Adv. 6:43205–43216
Ma X, Lv G, Cheng X, Li W, Sang R, Zhang Y, Wang Q, Hai L, Wu Y (2017) Appl Organometal Chem. doi:10.1002/aoc.3854
Guo Y, Li J, Shi X, Liu Y, Xie K, Liu Y, Jiang Y, Yang B, Yang R (2017) Appl Organometal Chem. doi:10.1002/aoc.3592
Paul S, Islamc MM, Islam SM (2015) RSC Adv 5:42193–42221
Jia X, Yang D, Zhang S, Cheng J (2009) Org Lett 11:4716–4719
Lyons TW, Hull KL, Sanford MS (2011) J Am Chem Soc 133:4455–4464
Hiraki K, Fuchita Y (1981) Inorg Synth 26:208–211
Hiraki K, Fuchita Y, Takechi K (1981) Inorg Chem 20:4316–4320
Albert J, Granell J, Zafrilla J, Font-Bardia M, Solans X (2005) J Organomet Chem 690:422–429
Baba K, Tobisu M, Chatani N (2013) Angew Chem Int Ed 52:11892–11895
Powers DC, Ritter T (2009) Nat Chem 1:302–310
Powers DC, Geibel MAL, Klein JEMN, Ritter T (2009) J Am Chem Soc 131:17050–17051
Berry JF, Bill E, Bothe E, Cotton A, Dalal NS, Ibragimov SA, Kaur N, Liu CY, Murillo CA, Nellutla S, North M, Villagrán D (2007) J Am Chem Soc 129:1393–1401
Cotton FA, Koshevoy LO, Lahuerta P, Murillo CA, Sanaú M, Ubeda MA, Zhao Q (2006) J Am Chem Soc 2006(128):13674–13675
Kullberg ML, Lemke FR, Powell DR, Kubiak CP (1985) Inorg Chem 24:3589–3594
Holloway RG, Penfold BR (1976) J Chem Soc Chem Commun 203:485–486
Lide DR (2005) CRC handbook of chemistry and physics (Internet Version). http://www.hbcpnetbase.com, CRC Press, Boca Raton
Vicente J, Arcas A, Bautista D, Jones PG (1997) Organometallics 16:2127–2138
Veljkovi DŽ, Janjić GV, Zarić SD (2011) Cryst Eng Commun 13(2011):5005–5010
Steiner T (2003) Crystallogr Rev 9:177–228
Steiner T, Desiraju GR (1998) Chem Commun 8:891–892
Maluenda I, Navarro O (2015) Molecules 20:7528–7557
Suzuki A (1999) J Organomet Chem 576:147–168
Huynh HV, Chew YX (2010) Inorg Chim Acta 363:1979–1983
Huynh HV, Wu J (2009) J Organomet Chem 694:323–331
Chen C, Qiu HY, Chen WZ (2012) J Organomet Chem 696:4166–4172
Zhang XM, Xie WL, Chen WZ (2010) Tetrahedron 66:1188–1195
Schaaf PAVD, Kolly R, Tinkl M (2004) U.S. patent 20040167018
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) J Appl Cryst 42:339–341
Acknowledgements
The authors thank Beijing Natural Science Foundation (Grant No. 2164057) and National Natural Science Foundation of China (Grant No. 21502122) for financial support. In particular, we thank Dr. Wei Wei and Dr. Deng Xuebin for refining the X-ray molecular structures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, X., Wang, H., Yuan, J. et al. Palladacycles incorporating a carboxylate-functionalized phosphine ligand: syntheses, characterization and their catalytic applications toward Suzuki couplings in water. Transit Met Chem 42, 727–738 (2017). https://doi.org/10.1007/s11243-017-0181-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-017-0181-5