Abstract
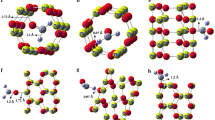
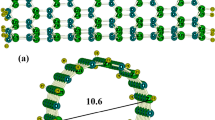
Using density functional theory, we have investigated the adsorption of formaldehyde (H2CO) on the interior and exterior walls of a carbon nitride nanotube (CNNT) in terms of energetic, geometric, and electronic properties. It was found that the adsorption is more preferential on the exterior surface of the tube with maximum adsorption energy of −7.4 kcal/mol. It has also been found that the adsorption energy per molecule is increased by increasing the number of adsorbed molecules. The results reveal that the electronic properties of CNNT are very sensitive to the presence of formaldehyde so that the HOMO/LUMO gap is reduced from 4.02 eV in the free tube to 2.44 eV in the most stable configuration of 3H2CO/CNNT complex. Also, we have showed that the response of the tube may depend on concentration of the H2CO molecules, suggesting that the CNNT might produce an electrical signal in the presence of H2CO molecules.





Similar content being viewed by others
References
Ayers GP, Gillett RW, Granek H, Deserves C, Cox RA (1997) Geophys Res Lett 24:401
Dingle P, Franklin P (2002) Ind Built Environ 11:111
Mine Y, Melander N, Richter D, Lancaster DG, Petrov KP, Tittel FK (1997) Appl Phys B 65:771
Beheshtian J, Peyghan AA, Bagheri Z (2012) Sens Actuators B Chem 171–172:846
Hamadanian M, Khoshnevisan B, Fotooh F (2011) Struct Chem 22:1205
Beheshtian J, Baei MT, Bagheri Z, Peyghan AA (2012) Microelectron J 43:452
Beheshtian J, Bagheri Z, Kamfiroozi M, Ahmadi A (2012) Struct Chem 23:653
Ahmadi A, Kamfiroozi M, Beheshtian J, Hadipour N (2011) Struct Chem 22:1261
Ahmadi Peyghan A, Omidvar A, Hadipour NL, Bagheri Z, Kamfiroozi M (2012) Physica E 44:1357
Turabekova MA, Dinadayalane TC, Leszczynsk D, Leszczynski J (2012) J Phys Chem C 116:6012
Saha S, Dinadayalane TC, Leszczynska D, Leszczynski J (2012) Chem Phys Lett 541:85
Saha S, Dinadayalane TC, Murray JS, Leszczynska D, Leszczynski J (2012) J Phys Chem C 116:22399
Goldoni A, Larciprete R, Petaccia L, Lizzit S (2003) J Am Chem Soc 125:11329
Collins PG, Bradley K, Ishigami M, Zettl A (2000) Science 287:1801
Burghard M (2005) Surf Sci Rep 58:1
Sun XH, Li CP, Wong WK, Wong NB, Lee CS, Lee ST, Teo BK (2002) J Am Chem Soc 124:14464
Zhang P, Crespi VH (2002) Phys Rev Lett 89:56403
Sorokin PB, Fedorov AS, Chernozatonskii LA (2006) Phys Solid State 48:398
Beheshtian J, Soleymanabadi H, Kamfiroozi M, Ahmadi A (2012) J Mol Model 18:2343
Beheshtian J, Ahmadi Peyghan A, Bagheri Z (2012) Physica E 44:1963
Baei MT, Peyghan AA, Bagheri Z (2012) Struct Chem. doi:10.1007/s11224-012-0129-5
Neidhardt J, Hultman L (2007) J Vac Sci Technol A 25:633
Kroke E, Schwarz M (2004) Coord Chem Rev 248:493
Cao C, Huang F, Cao C, Li J, Zhm H (2004) Chem Mater 16:5213
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S, Windus TL, Dupuis M, Montgomery JA (1993) J Comput Chem 14:1347
Oku T, Kuno M, Narita I (2004) J Phys Chem Solids 65:549
Erdogan R, Ozbek O, Onal I (2010) Surf Sci 604:1029
Tetasang S, Keawwangchai S, Wanno B, Ruangpornvisuti V (2012) Struct Chem 23:7
Contreras M, Avila D, Alvarez J, Rozas R (2010) Struct Chem 21:573
Lawson DB, Walker A (2012) Comput Theor Chem 981:31
Beheshtian J, Kamfiroozi M, Bagheri Z, Ahmadi A (2012) Comput Mater Sci 54:115
Beheshtian J, Kamfiroozi M, Bagheri Z, Peyghan AA (2012) Chin J Chem Phys 25:60
Olmsted J, Williams GM (1997) Chemistry: the molecular science. WCB, Iowa
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beheshtian, J., Peyghan, A.A. & Bagheri, Z. Formaldehyde adsorption on the interior and exterior surfaces of CN nanotubes. Struct Chem 24, 1331–1337 (2013). https://doi.org/10.1007/s11224-012-0172-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-012-0172-2