Abstract
Patient-Reported Outcome Measures (PROMs) are important tools to assess outcomes relevant to patients, with Health-Related Quality Of Life (HRQOL) as an important construct to be measured. Many different HRQOL PROMs are used in the type 2 diabetes field, however a complete overview of these PROMs is currently lacking. We therefore aimed to systematically describe and classify the content of all PROMs that have specifically been developed or validated to measure (aspects of) HRQOL in people with type 2 diabetes. A literature search was performed in PubMed and EMBASE until 31 December 2021. Studies on the development or validation of a PROM measuring HRQOL, or aspects of HRQOL, in people with type 2 diabetes were included. Title and abstract and full-text screening were conducted by two independent researchers and data extraction was performed independently by one of the researchers. Data were extracted on language in which the PROM was developed, target population, construct(s) being measured, names of (sub)scales and number of items per (sub)scale. In addition, all PROMs and subscales were classified according to specific aspects of HRQOL based on the Wilson & Cleary model (symptom status, functional status, general health perceptions) to aid researchers in PROM selection. In total 220 studies were identified that developed or validated PROMs that measure (aspects of) HRQOL in people with type 2 diabetes. Of the 116 unique HRQOL PROMs, 91 (of the subscales) measured symptom status, 60 measured functional status and 26 measured general health perceptions. In addition, 16 of the PROMs (subscales) measured global quality of life. 61 of the 116 PROMs (subscales) also include characteristics of the individual (e.g. aspects of personality, coping) or environment (e.g. social or financial support) and patient-reported experience measures (PREMs, e.g. measure of a patient's perception of their personal experience of the healthcare they have received, e.g. treatment satisfaction), which are not part of the HRQOL construct. Only 9 of the 116 PROMs measure all aspects of HRQOL based on the Wilson & Cleary model. Finally, 8 of the 116 PROMs stating to measure HRQOL, measured no HRQOL construct. In conclusion, a large number of PROMs are available for people with type 2 diabetes, which intend to measure (aspects of) HRQOL. These PROMs measure a large variety of (sub)constructs, which are not all HRQOL constructs, with a small amount of PROMs not measuring HRQOL at all. There is a need for consensus on which aspects of HRQOL should be measured in people with type 2 diabetes and which PROMs to use in research and daily practice. PROSPERO: CRD42017071012. COMET database: http://www.comet-initiative.org/studies/details/956.
Similar content being viewed by others
1 Introduction
Due to the high global prevalence of type 2 diabetes (˜400 million) combined with the chronic nature of the disease, it is important to measure outcomes that matter most to patients [1, 2]. This can be done by measuring patient-reported outcomes (PROs). PROs are health outcomes directly reported by patients about how they feel or function in relation to a health condition. In clinical research and care an important PRO to measure is (aspects of) health-related quality of life (HRQOL), including symptom status, functional status and general health perceptions [3]. The terms HRQOL and Quality of Life (QOL) are often used interchangeably. However many authors state that (overall) QOL is a broader concept, referring to how happy or satisfied a person is with his/her life as a whole [4,5,6]. Clinicians and researchers in the medical field generally prefer to measure only those aspects of QOL related to health (often referred to as HRQOL) instead of QOL, because the non-medical aspects of QOL are outside the scope of health care interventions. Not only in care, but also clinical trials, the measurement of HRQOL is becoming increasingly important.
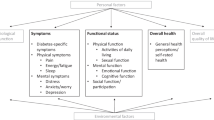
One of the most often used conceptual models of HRQOL was developed by Wilson and Cleary [4]. The model contains five levels of outcomes, namely biological and psychological variables, symptom status (including disease specific symptoms, physical symptoms and mental symptoms), functional status (including physical function, psychological function and social/role function), general health perceptions and overall quality of life (including overall quality of life, well-being and life satisfaction). In this review, we define HRQOL as symptoms, functional status and general health perceptions.
To date, many different PRO measurement instruments (PROMs) are available that measure HRQOL in people with type 2 diabetes, identified by previous reviews [7,8,9,10,11,12,13,14,15,16]. However, these reviews included studies in both people with type 1 and 2 diabetes, which represent different pathologies and large differences in age, and therefore different PROs may be relevant or the validity and reliability of PROMs may be different in people with type 1 versus type 2 diabetes [7, 8, 11]. Other reviews only included patients with amputations [14], only PROMs measuring one aspect of HRQOL, e.g. depressive symptoms [11] or were conducted over 10 years ago [9, 12]. A recent review by Wee et al. 2021 aimed to identify all PROMs used for people with diabetes [15]. However, Wee et al. did not classify (subscales of) PROMs according to which specific aspects of HRQOL, based on the Wilson & Cleary model, they measure. This classification is important because instrument selection should be based on the relevant aspects of HRQOL to measure, not on available PROMs, which are mostly multi-dimensional instruments that measure many different things. Therefore, the content and quality of PROMs should be evaluated for each PROM separately. Furthermore, often questionnaires that are being referred to as HRQOL PROMs include (subscales) that measure non-HRQOL aspects, such as characteristics of the individual, overall quality of life, or even patient-reported experience measures (PREMs), which are not part of the HRQOL construct according to the Wilson and Cleary model. This has not been made clear in previous reviews. Because of these research gaps, we aimed to systematically describe and classify the content of all PROMs that have specifically been developed or validated to measure (aspects of) HRQOL in people with type 2 diabetes.
2 Methods
This systematic review has been conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [17] and the COSMIN guideline for conducting systematic reviews [18]. The protocol was registered in the PROSPERO database on 2 July 2017 (registration number CRD42017071012).
2.1 Literature search
The databases PubMed and EMBASE were searched from date of inception until May 2019 and then updated until 31st of December 2021. This literature search has been performed by researcher CBT in cooperation with a medical librarian from the Amsterdam UMC, Amsterdam, the Netherlands. The search strategy was built up around three blocks of search terms, namely type 2 diabetes, measurement properties (i.e. different search terms for reliability, validity, responsiveness and interpretability) and PROMs (i.e. different search terms for report, questionnaire and survey). For the type 2 diabetes dimension search terms were used to identify studies that focused on people with type 2 diabetes. For finding studies on measurement properties a highly sensitive validated search filter was used [19] and a comprehensive PROM filter, developed by the Patient Reported Outcomes Measurement Group, University of Oxford and available through the COSMIN website, was used to search for PROMs [20]. An overview of the search strategy can be found in Appendix 1.
All identified studies were uploaded in Covidence [21], which is an online platform that supports researchers in conducting systematic reviews by enabling them to upload all of the identified studies, screening of the studies on title and abstract and full-text, resolve disagreements, and export data. Covidence was used during the study to remove duplicates and for the screening and selection process of the retrieved studies.
3 Study selection
Pairs of two researchers (JWB, PJME, AAH, IH, MLG, GM, CACP, FR, CBT and MW) independently reviewed the identified studies based on title and abstract and full-text article. In case of any disagreements between two of the researchers a third researcher was consulted to reach consensus. From the identified studies reference lists of the included articles were checked by one of the researchers (MLG or FR) to search for additional eligible studies, after which pairs of researchers reviewed the studies found through reference search. The screening and selection process was conducted based on pre-defined eligibility criteria.
A study was included when it met all five of the following inclusion criteria: (I) the authors aimed to develop a PROM, evaluate the measurement properties or evaluate the interpretability (e.g. floor and ceiling effects) of a PROM, (II) it concerned a PROM that aims (according to the authors of the included papers) to measure at least (aspects of) symptom status, functional status, general health perceptions or HRQOL based on the model of Wilson and Cleary [4], (III) the PROM is filled in by the patient in self-report, interview or diary form or is completed on behalf of the patient (proxy), (IV) > 50% of the study population consisted of people with type 2 diabetes, as reported in the article or when it could be assumed based on age and type of diabetes medication, or studies that reported measurement properties specifically for a subgroup of people with type 2 diabetes, and (V) the article is available in full-text. There were no restrictions on language in which the article was written.
A study was excluded when any of the following exclusion criteria were met: the PROM (I) was only used as a determinant or outcome measure or was used as a comparison instrument in a validation study of another instrument, (II) solely measured characteristics of the individual or behaviors (e.g. aspects of personality, self-efficacy, coping and eating behavior), characteristics of the environment (e.g. social support and financial support), patient-reported experience measures (PREM, i.e. a measure of a patient's perception of their personal experience of the healthcare they have received, e.g. treatment satisfaction) or overall quality of life (QOL) (e.g. well-being or satisfaction with life in general), or (III) was primarily developed for screening, diagnostic or prognostic purposes. PROMs that measure a combination of (aspects of) HRQOL as well as other constructs were included if the main aim was to measure (aspects of) HRQQL.
3.1 Data synthesis
Information from the included studies was systematically synthesized by one of the researchers (LG, MLG or FR). In case of any uncertainties a second researcher (CBT) was consulted. The characteristics of the PROM, including official name, language in which the PROM was developed, target population for which the PROM was developed (including type 1 or type 2 diabetes), construct(s) being measured, name of (sub)scales as well as number of items were extracted using a study-specific and pilot-tested PROM characteristics table. If necessary, relevant comments were also recorded. With regard to the (sub)scales, we extracted the number of items per subscale and the original names when possible. However, some studies did not clearly mention the number of items per subscale or the names of the subscales and then we noted the total number of items and for the names we either used a name that matched the authors’ description of the subscales or when the authors added or eliminated only a few items (not changing the scales), we used the subscale name of the original instrument.
All PROM (subscales) were classified according to the constructs of HRQOL measured, based on the Wilson & Cleary model [4]. This classification was based on reviewing the names of the (sub)scales and not the content of the PROMs. Some (sub)scales did not measure aspects of HRQOL, but were classified as measures of overall quality of life (including well-being and life satisfaction), characteristics of the individual/environment or PREM. If information on PROM characteristics could not be found in the paper, additional resources such as other articles, Google (e.g. manuals or websites) or the PROQOLID database [22] were consulted.
4 Results
Figure 1 represents the flowchart of the screening and selection process.
4.1 Characteristics of the PROMs
A total of 116 unique HRQOL PROMs were identified, of which 82 (70.7%) were specifically developed for people with (type 1 and 2) diabetes (Table 1). Other PROMs were validated in people with type 2 diabetes, but were originally developed for 21 different target populations, the main one being the general population, namely 20/116 (17.2%). The PROMs were developed in 32 different languages, most often in English (N = 68), Dutch (N = 9), Japanese (N = 7) and Spanish (N = 7). 7/116 (6.0%) PROM were developed in more than one language at the same time, such as the World Health Organisation Quality of Life (WHOQOL-100) [23] and the World Health Organisation Quality of Life (WHOQOL)-BREF [24, 25]. For all 116 PROMs, the number of (sub-)scales varied from 1 to 21.
We identified numerous different versions of the same PROM, for example 17 different versions were identified for the Diabetes Quality of Life questionnaire (DQOL). For many PROMs, these versions arose from translations, which during the validation process were modified by removing items or adding new items. By modifying, this makes it a new PROM, because it cannot be assumed that measurement properties are the same for different versions. When PROMs were only translated, with the same amount of subscales and items per subscales, we tallied this PROM as one of the same version and added the reference to that row of the PROM in Table 1. Finally, two studies consisted of non-standard PROMs, which were a decision tree [26] and a visual interactive PROM [27].
4.2 Levels of HRQOL measured with the PROMs
Table 2 and Supplemental Table 1 provide an overview of the specific levels of HRQOL that the included PROMs measure based on the Wilson and Cleary model [4]. Of the 116 unique HRQOL PROMs, 91 of their subscales measured symptom status, 60 measured functional status and 26 measured general health perceptions. With regard to symptom status, 22/91 measured diabetes-related symptoms, which included problems with vision, hearing, speaking, neuropathy, hypoglycemia, hyperglycemia, motor agitation and vasomotor function disturbance as well as cardiovascular disease. When examining the PROMs, there is overlap between the diabetes-related symptoms subscales and the general symptom status scales referring to physical symptoms and mental symptoms, such as pain or depressive feelings. For example, the Patient-reported outcomes in Thai patients with type 2 diabetes mellitus (PRO-DM-Thai) states to measure diabetes-related symptoms, but these include sleep problems, sexual problems and pain, which could be considered generic symptoms [28].
Within the symptom status level, 31/91 of the PROMs (subscales) measured physical symptoms, including pain, energy/fatigue and sleep as well as 69/91 measured mental symptoms, including distress, anxiety/worry and depression. With regard to the functional status level, 40/60 of the PROMs measured physical function, including activities of daily living and sexual function, 28/60 measured psychological function and 38/60 measured social/role function. There is a lot of heterogeneity, for example in the social function level, with many different constructs being measured, such as social well-being, restriction of social function, social role fulfillment and psychosocial disabilities, but also having friends, work and relationships, alienation, barriers and social burden.
In addition, 16/116 of the PROMs measured global quality of life. 61/116 of the HRQOL PROMs also include characteristics of the individual or environment and even PREMs, rather than only aspects of HRQOL. This includes characteristics of the individual, for example positive attitude [29,30,31], characteristics of the environment such as financial situation [31,32,33,34] or PREMs, such as treatment satisfaction [28, 34,35,36,37]. For one PROM it was specifically mentioned that demographics were also assessed as part of the PROM, namely the Diabetes Quality of Life Clinical Trial Questionnaire (DQLCTQ) [35].
Finally, only 9/116 of the HRQOL PROMs measured all aspects of HRQOL based on the Wilson & Cleary model. These PROMs include the DQLCTQ [35], Health Status Questionnaire (HSQ) 2.0 [38], PRO-DM-Thai [28], Quality of Life for Indian diabetes Patients (QOLID) [34], Self-perception of health [39], 12-Item Short Form Health Survey (SF-12) [40,41,42], 20-item Short Form Health Survey (SF-20) [43], 36-Item Short Form Health Survey (SF-36) [29, 44,45,46,47,48,49,50,51,52,53,54,55] and Well-being Enquiry for Diabetics (WED) [56]. Also, despite the fact that the authors of the included papers claimed that the PROM aims to measure at least (aspects of) symptom status, functional status, general health perceptions or HRQOL, 8/116 of the PROMs measured only global quality of life or PREMs and no HRQOL construct(s).
5 Discussion
In our systematic review of the literature, from a total of 220 studies, we identified 116 unique PROMs aiming to measure (aspects of) HRQOL in people with type 2 diabetes. Of these HRQOL PROMs, 80% (of the subscales) measured symptom status, 50% measured functional status and 20% measured general health perceptions. In addition, 15% of the PROMs (subscales) measured global quality of life. 50% of the 116 PROMs (subscales) also include characteristics of the individual (e.g. aspects of personality, coping) or environment (e.g. social or financial support) and patient-reported experience measures (PREMs, e.g. measure of a patient's perception of their personal experience of the healthcare they have received, e.g. treatment satisfaction), which are not part of the HRQOL construct. The (sub-)scales of these PROMs thus presented a great heterogeneity of constructs, with about 5% of the PROMs measuring all aspects of HRQOL based on the Wilson & Cleary model and about 5% not measuring HRQOL (constructs) at all. This review shows the great amount of PROMs developed. Furthermore, some PROMs are very long, which may suggest poor acceptability.
When conducting this review we faced multiple challenges. First, the terminology used for the constructs the (subscales of the) PROMs measure was unclear and definitions of the constructs are mostly lacking. It was therefore unclear to us whether names of the PROMs and subscales represent different or the same concepts. This large variability in operationalization of HRQOL made it difficult to classify the PROMs. This lack of clarity about what a PROM actually measures also makes it difficult or even impossible to know whether a PROM has good validity (i.e. whether it measures what it is supposed to measure). A second challenge was that information regarding the characteristics of the PROMs was often lacking or misleading. For example, the availability and the number and names of (sub-)scales and the number of items per (sub-)scale were often not presented in the paper. As a result we had to consult additional resources, such as other articles, Google (e.g. manuals or websites) or the PROQOLID database [22]. However, even this strategy sometimes failed, which may have resulted in an incomplete overview of the PROMs (Table 1). This poor reporting is possibly due to older papers not meeting our modern day standards, but hampers researchers and health care providers to select the best PROM for their purpose. The poor information status and very large hetereogeneity in PROMs (subscales) is not unique to the diabetes field [243]. PROMs are increasingly used as primary outcome measures in studies and tools for clinical decision making. The poor state makes it very difficult, and potentially even impossible, to compare study results or cohorts directly, since all PROMs measure different constructs and thus different outcomes. In this review, we did not systematically evaluate the measurement properties of the PROMs, such as content validity, construct validity, reliability and responsiveness. Therefore, researchers should be careful when using this review to select PROMs as we cannot guarantee that the content of the PROMs or subscales really match the intended construct and we cannot guarantee that the PROMs are reliable and responsive to change [244].
This review highlights the great amount of PROMs developed and used and the heterogeneity of their content. We feel there is a need to reach consensus on which PROM to measure HRQOL as well as which HRQOL aspects are most important to measure for people with type 2 diabetes. One solution is the development of Core Outcome Sets (COS) or Standard Sets, which are agreed sets of outcomes (and associated measurement instruments) to be measured in all trials or clinical practice. International organizations such as COMET (https://www.comet-initiative.org/) and ICHOM (www.ichom.org) have developed such COSs for type 2 diabetes [245,246,247]. However, the value of these COSs are limited, because they have a strong focus on biological outcomes, such as glycemic control [199,200,201] and there was limited input from people with expertise in PRO measurement or people with type 2 diabetes. This resulted in dissimilar recommendations regarding PROMs between the initiatives, but also inclusion of the ‘Diabetes Treatment Satisfaction Questionnaire’ (which is a PREM) and only inclusion of activities of daily living and overall quality of life, and no other aspects of HRQOL [245,246,247]. Qualitative studies show the importance of ‘To live a good life with diabetes’ for people with type 2 diabetes [248].
6 Limitations and strengths
This systematic review has several limitations and strengths. The first limitation is that the classification of the constructs was made based on reviewing the names of (sub)scales and not their content. We acknowledge that this may have resulted in misclassification, because of misleading construct names that do not reflect the content. It would have been better to look at the content of the PROMs to determine what aspects of HRQOL they measure, rather than using the names of the instrument (scales). We have done so for part of the PROMs, i.e. only the disease-specific HRQOL PROMs, in a separate review [244] where we did a full content validity assessment of these PROMs. However, the fact that there might be a mismatch between our classification and what the PROMs actually measure is a striking finding of this review. It is problematic that the name and description of a PROM as published in the literature does not tell us, or may even mislead us about what the PROM actually measures. This strongly hampers researchers and clinicians to select the optimal PROM for their purpose. Second, even though using an extensive search string, we identified 27% of the included studies from reference lists. However, by using this extensive search strategy our review is more complete than previous reviews specifically on HRQOL in those with type 2 diabetes. For example, we identified over 50 HRQOL PROMs with our search that were not found in the Wee et al. review [15]. We speculate this discrepancy is due to their lack of reference checking. Strengths of this systematic review were the extensive search with no restrictions on publication data or language as well as reference checking. Second, the use of a conceptual model to assess which aspects of HRQOL were measured by PROM (subscales) provides helpful information for researchers and health care providers searching for a PROM to measure one or more specific aspects of HRQOL, that is not provided in previous reviews. As stated before, instrument selection should be based on which relevant aspects of HRQOL one wants to measure and different aspects of HRQOL can be measured with subscales from different PROMs. Even though the Wilson and Cleary model is the most frequently used, other conceptual models are available that might be preferred by other researchers [4]. However, our conclusion on the heterogeneity and lack of clarity of constructs being measured with PROMs in the diabetes field would not have been different. Finally, despite our systematic review providing an overview and identifying the difficulties of the field, it also provides caution and food for thought regarding the use of the PROMs. Future studies are needed to provide definitive recommendations on which PROMs to use in people with type 2 diabetes.
7 Conclusion
A large number of PROMs are available for people with type 2 diabetes, which intend to measure (aspects of) HRQOL. These PROMs measure a large variety of (sub)constructs, which are not all HRQOL constructs, with a small amount of PROMs not measuring HRQOL at all. There is a need for consensus on which aspects of HRQOL should be measured in people with type 2 diabetes and which PROMs to use in research and daily practice.
Abbreviations
- COS:
-
Core Outcome Sets
- HRQOL:
-
Health-Related Quality Of Life
- PRO:
-
Patient Reported Outcome
- PROM:
-
Patient Reported Outcome Measure
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- PREM:
-
Patient-Reported Experience Measures
- QOL:
-
Quality Of Life
References
Snyder CF, Jensen RE, Segal JB, Wu AW. Patient-reported outcomes (PROs): putting the patient perspective in patient-centered outcomes research. Med Care. 2013;51(8 Suppl 3):S73–9.
Wheat H, Horrell J, Valderas JM, Close J, Fosh B, Lloyd H. Can practitioners use patient reported measures to enhance person centred coordinated care in practice? A qualitative study. Health Qual Life Outcomes. 2018;16(1):223.
Squitieri L, Bozic KJ, Pusic AL. The Role of Patient-Reported Outcome Measures in Value-Based Payment Reform. Value Health. 2017;20(6):834–6.
Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273(1):59–65.
Valderas JM, Alonso J. Patient reported outcome measures: a model-based classification system for research and clinical practice. Qual Life Res. 2008;17(9):1125–35.
Karimi M, Brazier J. Health, Health-Related Quality of Life, and Quality of Life: What is the Difference? Pharmacoeconomics. 2016;34(7):645–9.
El Achhab Y, Nejjari C, Chikri M, Lyoussi B. Disease-specific health-related quality of life instruments among adults diabetic: A systematic review. Diabetes Res Clin Pract. 2008;80(2):171–84.
Garratt AM, Schmidt L, Fitzpatrick R. Patient-assessed health outcome measures for diabetes: a structured review. Diabet Med. 2002;19(1):1–11.
Luscombe FA. Health-related quality of life measurement in type 2 diabetes. Value Health. 2000;3(Suppl 1):15–28.
Roborel de Climens A, Tunceli K, Arnould B, Germain N, Iglay K, Norquist J, et al. Review of patient-reported outcome instruments measuring health-related quality of life and satisfaction in patients with type 2 diabetes treated with oral therapy. Curr Med Res Opin. 2015;31(4):643–65.
van Dijk SEM, Adriaanse MC, van der Zwaan L, Bosmans JE, van Marwijk HWJ, van Tulder MW, et al. Measurement properties of depression questionnaires in patients with diabetes: a systematic review. Qual Life Res. 2018;27(6):1415–30.
Vieta A, Badia X, Sacristan JA. A systematic review of patient-reported and economic outcomes: value to stakeholders in the decision-making process in patients with type 2 diabetes mellitus. Clin Ther. 2011;33(9):1225–45.
Chen YT, Tan YZ, Cheen M, Wee HL. Patient-Reported Outcome Measures in Registry-Based Studies of Type 2 Diabetes Mellitus: a Systematic Review. Curr Diab Rep. 2019;19(11):135.
Miller R, Ambler GK, Ramirez J, Rees J, Hinchliffe R, Twine C, et al. Patient Reported Outcome Measures for Major Lower Limb Amputation Caused by Peripheral Artery Disease or Diabetes: A Systematic Review. Eur J Vasc Endovasc Surg. 2020.
Wee PJL, Kwan YH, Loh DHF, Phang JK, Puar TH, Ostbye T, et al. Measurement Properties of Patient-Reported Outcome Measures for Diabetes: Systematic Review. J Med Internet Res. 2021;23(8): e25002.
Martin-Delgado J, Guilabert M, Mira-Solves J. Patient-Reported Experience and Outcome Measures in People Living with Diabetes: A Scoping Review of Instruments. Patient. 2021;14(6):759–73.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Prinsen CAC, Mokkink LB, Bouter LM, Alonso J, Patrick DL, de Vet HCW, et al. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual Life Res. 2018.
Terwee CB, Jansma EP, Riphagen II, de Vet HC. Development of a methodological PubMed search filter for finding studies on measurement properties of measurement instruments. Qual Life Res. 2009;18(8):1115–23.
COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) website. 2017 [Available from: www.cosmin.nl.
Covidence. 2021 [Available from: https://www.covidence.org/.
MapiResearchTrust. PROQOLID 2021 [Available from: https://eprovide.mapi-trust.org/.
Pibernik-Okanovic M. Psychometric properties of the World Health Organisation quality of life questionnaire (WHOQOL-100) in diabetic patients in Croatia. Diabetes Res Clin Pract. 2001;51(2):133–43.
Kolawole BA, Mosaku SK, Ikem RT. A comparison of two measures of quality of life of Nigerian clinic patients with type 2 diabetes mellitus. Afr Health Sci. 2009;9(3):161–6.
Sreedevi A, Cherkil S, Kuttikattu DS, Kamalamma L, Oldenburg B. Validation of WHOQOL-BREF in Malayalam and Determinants of Quality of Life Among People With Type 2 Diabetes in Kerala. India Asia Pac J Public Health. 2016;28(1 Suppl):62S-S69.
Garcia AA. The Diabetes Symptom Self-Care Inventory: development and psychometric testing with Mexican Americans. J Pain Symptom Manage. 2011;41(4):715–27.
Klis S, Vingerhoets AJ, de Wit M, Zandbelt N, Snoek FJ. Pictorial Representation of Illness and Self Measure Revised II (PRISM-RII): a novel method to assess perceived burden of illness in diabetes patients. Health Qual Life Outcomes. 2008;6:104.
Chuayruang K, Sriratanaban J, Hiransuthikul N, Suwanwalaikorn S. Development of an instrument for patient-reported outcomes in Thai patients with type 2 diabetes mellitus (PRO-DM-Thai). Asian Biomedicine. 2015;9(1):7–19.
Anderson RM, Fitzgerald JT, Wisdom K, Davis WK, Hiss RG. A comparison of global versus disease-specific quality-of-life measures in patients with NIDDM. Diabetes Care. 1997;20(3):299–305.
Fitzgerald JT, Davis WK, Connell CM, Hess GE, Funnell MM, Hiss RG. Development and validation of the Diabetes Care Profile. Eval Health Prof. 1996;19(2):208–30.
Abetz L, Sutton M, Brady L, McNulty P, Gagnon DD. The Diabetic Foot Ulcer Scale (DFS): a quality of life instrument for use in clinical trials. Practical Diabetes Int. 2002;19(6):167–75.
Holmes-Truscott E, Skovlund SE, Hendrieckx C, Pouwer F, Peyrot M, Speight J. Assessing the perceived impact of diabetes on quality of life: Psychometric validation of the DAWN2 Impact of Diabetes Profile in the second Diabetes MILES - Australia (MILES-2) survey. Diabetes Res Clin Pract. 2019;150:253–63.
Goh SG, Rusli BN, Khalid BA. Development and validation of the Asian Diabetes Quality of Life (AsianDQOL) Questionnaire. Diabetes Res Clin Pract. 2015;108(3):489–98.
Nagpal J, Kumar A, Kakar S, Bhartia A. The development of ’Quality of Life Instrument for Indian Diabetes patients (QOLID): a validation and reliability study in middle and higher income groups. J Assoc Physicians India. 2010;58:295–304.
Shen W, Kotsanos JG, Huster WJ, Mathias SD, Andrejasich CM, Patrick DL. Development and validation of the Diabetes Quality of Life Clinical Trial Questionnaire. Med Care. 1999;37(4 Suppl Lilly):AS45–66.
Araki A, Ito H. Development of elderly diabetes burden scale for elderly patients with diabetes mellitus. Geriatr Gerontol Int. 2003;3:212–24.
Oobe M, Tanaka M, Fuchigami M, Sakata T. Preparation of a quality of life (QOL) questionnaire for patients with type II diabetes and prospects for its clinical application. Fukuoka Igaku Zasshi. 2007;98(10):379–87.
Morgan B, Buscemi C, Fajardo V. Assessing Instruments in a Cuban American Population With Type 2 Diabetes Mellitus. Journal of transcultural nursing : official journal of the Transcultural Nursing Society / Transcultural Nursing Society. 2004;15:139–46.
Rao PR, Shobhana R, Lavanya A, Padma C, Vijay V, Ramachandran A. Development of a reliable and valid psychosocial measure of self-perception of health in type 2 diabetes. J Assoc Physicians India. 2005;53:689–92.
Maddigan SL, Feeny DH, Johnson JA, Investigators D. Construct validity of the RAND-12 and Health Utilities Index Mark 2 and 3 in type 2 diabetes. Qual Life Res. 2004;13(2):435–48.
Maurischat C, Herschbach P, Peters A, Bullinger M. Factorial validity of the Short Form 12 (SF-12) in patients with diabetes mellitus. Psychol Sci Q. 2008;50(1):7–20.
Wan EYF, Choi EPH, Yu EYT, Chin WY, Fung CSC, Chan AKC, et al. Evaluation of the internal and external responsiveness of Short Form-12 Health Survey version 2 (SF-12v2) in patients with type 2 diabetes mellitus. Qual Life Res. 2018;27(9):2459–69.
Westaway MS, Rheeder P, Gumede T. The effect of type 2 diabetes mellitus on health-related quality of life (HRQOL). Curationis. 2001;24(1):74–8.
Abbasi-Ghahramanloo A, Soltani-Kermanshahi M, Mansori K, Khazaei-Pool M, Sohrabi M, Baradaran HR, et al. Comparison of SF-36 and WHOQoL-BREF in Measuring Quality of Life in Patients with Type 2 Diabetes. Int J Gen Med. 2020;13:497–506.
Ahroni JH, Boyko EJ. Responsiveness of the SF-36 among veterans with diabetes mellitus. J Diabetes Complications. 2000;14(1):31–9.
Bagheri Z, Jafari P, Mahmoodi M, Dabbaghmanesh MH. Testing whether patients with diabetes and healthy people perceive the meaning of the items in the Persian version of the SF-36 questionnaire similarly: a differential item functioning analysis. Qual Life Res. 2017;26(4):835–45.
Glasziou P, Alexander J, Beller E, Clarke P, Group AC. Which health-related quality of life score? A comparison of alternative utility measures in patients with Type 2 diabetes in the ADVANCE trial. Health Qual Life Outcomes. 2007;5:21.
Hirsch A, Bartholomae C, Volmer T. Dimensions of quality of life in people with non-insulin-dependent diabetes. Qual Life Res. 2000;9(2):207–18.
Hu J, Gruber K, Hsueh K-H. Psychometric properties of the Chinese version of the SF-36 in older adults with diabetes in Beijing. China Diabetes research and clinical practice. 2010;88:273–81.
Huang IC, Hwang CC, Wu MY, Lin W, Leite W, Wu AW. Diabetes-specific or generic measures for health-related quality of life? Evidence from psychometric validation of the D-39 and SF-36. Value Health. 2008;11(3):450–61.
Jacobson AM, de Groot M, Samson JA. The evaluation of two measures of quality of life in patients with type I and type II diabetes. Diabetes Care. 1994;17(4):267–74.
Linzer M, Pierce C, Lincoln E, Miller DR, Payne SM, Clark JA, et al. Preliminary validation of a patient-based self-assessment measure of severity of illness in type 2 diabetes: results from the pilot phase of the Veterans Health Study. J Ambul Care Manage. 2005;28(2):167–76.
Martin ML, Patrick DL, Gandra SR, Bennett AV, Leidy NK, Nissenson AR, et al. Content validation of two SF-36 subscales for use in type 2 diabetes and non-dialysis chronic kidney disease-related anemia. Qual Life Res. 2011;20(6):889–901.
Woodcock AJ, Julious SA, Kinmonth AL, Campbell MJ, Diabetes Care From Diagnosis G. Problems with the performance of the SF-36 among people with type 2 diabetes in general practice. Qual Life Res. 2001;10(8):661–70.
Yordanova S, Petkova V, Petrova G, Dimitrov M, Naseva E, Dimitrova M, et al. Comparison of health-related quality-of-life measurement instruments in diabetic patients. Biotechnol Biotechnol Equip. 2014;28(4):769–74.
Mannucci E, Ricca V, Bardini G, Rotella CM. Well-being Enquiry for Diabetics: A new measure of diabetes-related quality of life. Diab Nutr Metab. 1996;9(2):89–102.
Akinci F, Yildirim A, Ogutman B, Ates M, Gozu H, Deyneli O, et al. Translation, cultural adaptation, initial reliability, and validation of Turkish 15D’s version: a generic health-related quality of life (HRQoL) instrument. Eval Health Prof. 2005;28(1):53–66.
Bradley C, Todd C, Gorton T, Symonds E, Martin A, Plowright R. The development of an individualized questionnaire measure of perceived impact of diabetes on quality of life: the ADDQoL. Qual Life Res. 1999;8(1–2):79–91.
Demirci H, Cinar Y, Bayram N, Bilgel N. Quality of life in type II diabetic patients in primary health care. Dan Med J. 2012;59(10):A4468.
Lemon SC, Rosal MC, Welch G. Measuring quality of life in low-income, Spanish-speaking Puerto Ricans with type 2 diabetes residing in the mainland U.S. Qual Life Res. 2011;20(9):1507–11.
Speight J, Sinclair AJ, Browne JL, Woodcock A, Bradley C. Assessing the impact of diabetes on the quality of life of older adults living in a care home: validation of the ADDQoL Senior. Diabet Med. 2013;30(1):74–80.
Costa FA, Guerreiro JP, Duggan C. An Audit of Diabetes Dependent Quality of Life (ADDQoL) for Portugal: exploring validity and reliability. Pharm Pract (Granada). 2006;4(3):123–8.
Daher AM, AlMashoor SH, Winn T. Performance of the Malay Audit of Diabetes Dependent Quality of Life-18 and Associates of Quality of Life among Patients with Type 2 Diabetes Mellitus from Major Ethnic Groups of Malaysia. PLoS ONE. 2016;11(10): e0163701.
Kamarul Imran M, Ismail AA, Naing L, Wan Mohamad WB. The reliability and validity of the Malay version of the 18-item audit of Diabetes Dependent Quality of Life (the Malay ADDQOL) questionnaire. Southeast Asian J Trop Med Public Health. 2007;38(2):398–405.
Abbatecola AM, Spazzafumo L, Fabbietti P, Testa R, Rabini RA, Bonfigli AR, et al. Diabetes-related quality of life is enhanced by glycaemic improvement in older people. Diabet Med. 2015;32(2):243–9.
Bak E, Marcisz C, Nowak-Kapusta Z, Dobrzyn-Matusiak D, Marcisz E, Krzeminska S. Psychometric properties of the Audit of Diabetes-Dependent Quality of Life (ADDQoL) in a population-based sample of Polish adults with type 1 and 2 diabetes. Health Qual Life Outcomes. 2018;16(1):53.
Fung CS, Wan EY, Yu CL, Wong CK. Validity and reliability of the 19-item Audit of Diabetes-Dependent Quality of Life (ADDQoL-19) questionnaire in Chinese patients with type 2 diabetes mellitus in primary care. Qual Life Res. 2016;25(9):2373–8.
Jannoo Z, Yap BW, Musa KI, Lazim MA, Hassali MA. An audit of diabetes-dependent quality of life in patients with type 2 diabetes mellitus in Malaysia. Qual Life Res. 2015;24(9):2297–302.
Kong D, Ding Y, Zuo X, Su W, Xiu L, Lin M, et al. Adaptation of the Audit of Diabetes-Dependent Quality of Life questionnaire to people with diabetes in China. Diabetes Res Clin Pract. 2011;94(1):45–52.
Magwood G, Jenkins C, Zapka J. Validation of Diabetes Health-Related Quality-of-Life Instruments Using Cognitive Interviewing With Older African Americans. J Nurs Meas. 2009;17:195–220.
Soon SS, Goh SY, Bee YM, Poon JL, Li SC, Thumboo J, et al. Audit of Diabetes-Dependent Quality of Life (ADDQoL) [Chinese Version for Singapore] questionnaire: reliability and validity among Singaporeans with type 2 diabetes mellitus. Appl Health Econ Health Policy. 2010;8(4):239–49.
Turk E, Prevolnik Rupel V, Tapajner A, Isola A. Reliability and Validity of the Audit on Diabetes-Dependent Quality of Life (ADDQoL) and EQ-5D in Elderly Slovenian Diabetes Mellitus Type 2 Patients. Health. 2014;6:699–711.
Visockiene Z, Narkauskaite-Nedzinskiene L, Puronaite R, Mikaliukstiene A. Validation of the LITHUANIAN version of the 19-item audit of diabetes dependent quality of life (ADDQOL - LT) questionnaire in patients with diabetes. Health Qual Life Outcomes. 2018;16(1):206.
Wee HL, Tan CE, Goh SY, Li SC. Usefulness of the Audit of Diabetes-Dependent Quality-of-Life (ADDQoL) questionnaire in patients with diabetes in a multi-ethnic Asian country. Pharmacoeconomics. 2006;24(7):673–82.
Zhang XH, Tan K, Tan HH, Thumboo J, Li SC. Are English and Chinese Versions of the Audit of Diabetes-Dependent Quality of Life Equivalent? An Exploratory Study Based on the Universalist Approach. Value Health Reg Issues. 2012;1(1):75–81.
Elasy TA, Samuel-Hodge CD, DeVellis RF, Skelly AH, Ammerman AS, Keyserling TC. Development of a health status measure for older African-American women with type 2 diabetes. Diabetes Care. 2000;23(3):325–9.
Hayes RP, Nelson DR, Meldahl ML, Curtis BH. Ability to perform daily physical activities in individuals with type 2 diabetes and moderate obesity: a preliminary validation of the Impact of Weight on Activities of Daily Living Questionnaire. Diabetes Technol Ther. 2011;13(7):705–12.
Hayes RP, Schultz EM, Naegeli AN, Curtis BH. Test-retest, responsiveness, and minimal important change of the ability to perform physical activities of daily living questionnaire in individuals with type 2 diabetes and obesity. Diabetes Technol Ther. 2012;14(12):1118–25.
Torres HC, Virginia AH, Schall VT. Validation of Diabetes Mellitus Knowledge (DKN-A) and Attitude (ATT-19) Questionnaires. Rev Saude Publica. 2005;39(6):906–11.
Welch G, Beeney LJ, Dunn SM. The development of the diabetes integration scale: A psychometric study of the ATT39. Multivar Exp Clin Res. 1996;11(2):75–88.
Dunn SM, Smartt HH, Beeney LJ, Turtle JR. Measurement of emotional adjustment in diabetic patients: validity and reliability of ATT39. Diabetes Care. 1986;9(5):480–9.
Ting RZ, Nan H, Yu MW, Kong AP, Ma RC, Wong RY, et al. Diabetes-related distress and physical and psychological health in chinese type 2 diabetic patients. Diabetes Care. 2011;34(5):1094–6.
Carter J, Cogo-Moreira H, Herrmann N, Merino D, Yang P, Shah BR, et al. Validity of the Center for Epidemiological Studies Depression scale in Type 2 diabetes. J Psychosom Res. 2016;90:91–7.
Lehmann V, Makine C, Karsidag C, Kadioglu P, Karsidag K, Pouwer F. Validation of the Turkish version of the Centre for Epidemiologic Studies Depression Scale (CES-D) in patients with type 2 diabetes mellitus. BMC Med Res Methodol. 2011;11:109.
McHale M, Hendrikz J, Dann F, Kenardy J. Screening for depression in patients with diabetes mellitus. Psychosom Med. 2008;70(8):869–74.
Rankin SH, Galbraith ME, Johnson S. Reliability and validity data for a Chinese translation of the Center for Epidemiological Studies-Depression. Psychol Rep. 1993;73(3 Pt 2):1291–8.
Zhang Y, Ting RZW, Lam MHB, Lam S-P, Yeung RO, Nan H, et al. Measuring depression with CES-D in Chinese patients with type 2 diabetes: the validity and its comparison to PHQ-9. BMC Psychiatry. 2015;15(1):198.
Hsu LF, Kao CC, Wang MY, Chang CJ, Tsai PS. Psychometric testing of a Mandarin Chinese Version of the Clinically Useful Depression Outcome Scale for patients diagnosed with type 2 diabetes mellitus. Int J Nurs Stud. 2014;51(12):1595–604.
Price P, Harding K. Cardiff Wound Impact Schedule: the development of a condition-specific questionnaire to assess health-related quality of life in patients with chronic wounds of the lower limb. Int Wound J. 2004;1(1):10–7.
Jaksa PJ, Mahoney JL. Quality of life in patients with diabetic foot ulcers: validation of the Cardiff Wound Impact Schedule in a Canadian population. Int Wound J. 2010;7(6):502–7.
Fagerdahl AM, Bostrom L, Ulfvarson J, Bergstrom G, Ottosson C. Translation and validation of the wound-specific quality of life instrument Cardiff Wound Impact Schedule in a Swedish population. Scand J Caring Sci. 2014;28(2):398–404.
Sriyani KA, Gunawardena N, Wasalathanthri S, Hettiarachchi P. Validation of Sinhala Version of Cardiff Wound Impact Schedule in Patients with Diabetic Leg and Foot Ulcers. Asian Nurs Res (Korean Soc Nurs Sci). 2016;10(3):240–5.
Granado-Casas M, Martinez-Gonzalez D, Martinez-Alonso M, Doria M, Alcubierre N, Valls J, et al. Psychometric Validation of the Cardiff Wound Impact Schedule Questionnaire in a Spanish Population with Diabetic Foot Ulcer. J Clin Med. 2021;10(17).
Huang Y, Wu M, Xing P, Xie T, Cao Y, Qian P, et al. Translation and validation of the Chinese Cardiff Wound Impact Schedule. Int J Low Extrem Wounds. 2014;13(1):5–11.
Boyer JG, Earp JA. The development of an instrument for assessing the quality of life of people with diabetes. Diabetes-39. Med Care. 1997;35(5):440–53.
Khader YS, Bataineh S, Batayha W. The Arabic version of Diabetes-39: psychometric properties and validation. Chronic Illn. 2008;4(4):257–63.
Lopez-Carmona JM, Rodriguez-Moctezuma R. Adaptation and validation of quality of life instrument Diabetes 39 for Mexican patients with type 2 diabetes mellitus. Salud Publica Mex. 2006;48(3):200–11.
Nguyen TQ, Vo TQ, Nguyen GH, Nguyen TD. Assessment of Health-Related Quality of Life in Patients with Type II Diabetes Mellitus: A Population-Based Study at a Tertiary Hospital. Journal of Clinical and Diagnostic Research. 2018;12(6):LC44-LC51.
Queiroz FA, Pace AE, Santos CB. Cross-cultural adaptation and validation of the instrument Diabetes - 39 (D-39): brazilian version for type 2 diabetes mellitus patients - stage 1. Rev Lat Am Enfermagem. 2009;17(5):708–15.
Leite WL, Huang IC, Marcoulides GA. Item Selection for the Development of Short Forms of Scales Using an Ant Colony Optimization Algorithm. Multivariate Behav Res. 2008;43(3):411–31.
Li J, Li Z, Zhao W, Pan H, Halloran EJ. The Reliability and Validity of the Diabetes Care Profile for Chinese Populations. Eval Health Prof. 2015;38(2):200–18.
Sousa VD, Zanetti ML, Zauszniewski JA, Mendes IA, Daguano MO. Psychometric properties of the Portuguese version of the Depressive Cognition Scale in Brazilian adults with diabetes mellitus. J Nurs Meas. 2008;16(2):125–35.
Zauszniewski JA, Chung C, Krafcik K, Sousa VD. Psychometric testing of the depressive cognition scale in women with type 2 diabetes. J Nurs Meas. 2001;9(1):61–72.
Sato E, Suzukamo Y, Miyashita M, Kazuma K. Development of a diabetes diet-related quality-of-life scale. Diabetes Care. 2004;27(6):1271–5.
Sato E, Ochiai R, Shibayama T, Nishigaki M, Abe Y, Sawa T, et al. Reliability and validity of revised and short form versions of diabetes diet-related quality of life scale. Diabetol Int. 2017;8(2):181–92.
Fisher L, Glasgow RE, Mullan JT, Skaff MM, Polonsky WH. Development of a brief diabetes distress screening instrument. Ann Fam Med. 2008;6(3):246–52.
Chew BH, Mukhtar F, Sherina MS, Paimin F, Hassan NH, Jamaludin NK. The reliability and validity of the Malay version 17-item Diabetes Distress Scale. Malays Fam Physician. 2015;10(2):22–35.
Chin YW, Lai PS, Chia YC. The validity and reliability of the English version of the diabetes distress scale for type 2 diabetes patients in Malaysia. BMC Fam Pract. 2017;18(1):25.
Curcio R, Costa Alexandre NM, de Carvalho TH, Lima MHM. Translation and adaptation of the “Diabetes Distress Scale - DDS” in Brazilian culture. Acta Paul Enferm. 2012;25(5):762–7.
Farm BAS, Perwitasari DA, Thobari JA, Cao Q, Krabbe PFM, Postma MJ. Translation, Revision, and Validation of the Diabetes Distress Scale for Indonesian Type 2 Diabetic Outpatients with Various Types of Complications. Value Health Reg Issues. 2017;12:63–73.
Fenwick EK, Rees G, Holmes-Truscott E, Browne JL, Pouwer F, Speight J. What is the best measure for assessing diabetes distress? A comparison of the Problem Areas in Diabetes and Diabetes Distress Scale: results from Diabetes MILES-Australia. J Health Psychol. 2018;23(5):667–80.
Graue M, Haugstvedt A, Wentzel-Larsen T, Iversen MM, Karlsen B, Rokne B. Diabetes-related emotional distress in adults: reliability and validity of the Norwegian versions of the Problem Areas in Diabetes Scale (PAID) and the Diabetes Distress Scale (DDS). Int J Nurs Stud. 2012;49(2):174–82.
Martinez-Vega IP, Doubova SV, Aguirre-Hernandez R, Infante-Castaneda C. Adaptation and validation of the Distress Scale for Mexican patients with type 2 diabetes and hypertension: a cross-sectional survey. BMJ Open. 2016;6(3): e009723.
Mocan AS, Bāban A. An useful tool for diabetes emotional distress assessment> Validation of the Romanian version of the diabetes distress scale. Rom J Diabetes Nutr Metab Dis. 2015;22(4):425–31.
Polonsky WH, Fisher L, Earles J, Dudl RJ, Lees J, Mullan J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28(3):626–31.
Batais MA, Alosaimi FD, AlYahya AA, Aloofi OA, Almashouq MK, Alshehri KS, et al. Translation, cultural adaptation, and evaluation of the psychometric properties of an Arabic diabetes distress scale: A cross sectional study from Saudi Arabia. Saudi Med J. 2021;42(5):509–16.
Krzeminska S, Bak E. Psychometric Properties of the Polish Version of the Diabetes Distress Scale (DDS). Psychol Res Behav Manag. 2021;14:1149–56.
Thanakwang K, Thinganjana W, Konggumnerd R. Psychometric properties of the Thai version of the Diabetes Distress Scale in diabetic seniors. Clin Interv Aging. 2014;9:1353–61.
Kokoszka A. Depression in Diabetes Self-Rating Scale: a screening tool. Diabet Dośw i Klin. 2008;8:43–7.
Poole JL, Gonzales I, Tedesco T. Self-reports of hand function in persons with diabetes. Occup Ther Health Care. 2010;24(3):239–48.
Bann CM, Fehnel SE, Gagnon DD. Development and validation of the Diabetic Foot Ulcer Scale-short form (DFS-SF). Pharmacoeconomics. 2003;21(17):1277–90.
Hui LF, Yee-Tak Fong D, Yam M, Yuk IW. Translation and validation of the chinese diabetic foot ulcer scale - short form. Patient. 2008;1(2):137–45.
Macioch T, Sobol E, Krakowiecki A, Mrozikiewicz-Rakowska B, Kasprowicz M, Hermanowski T. Health related quality of life in patients with diabetic foot ulceration - translation and Polish adaptation of Diabetic Foot Ulcer Scale short form. Health Qual Life Outcomes. 2017;15(1):15.
Martinez-Gonzalez D, Doria M, Martinez-Alonso M, Alcubierre N, Valls J, Verdu-Soriano J, et al. Adaptation and Validation of the Diabetic Foot Ulcer Scale-Short Form in Spanish Subjects. J Clin Med. 2020;9(8).
Kontodimopoulos N, Veniou A, Tentolouris N, Niakas D. Validity and reliability of the Greek version of the Diabetic Foot Ulcer Scale - Short Form (DFS-SF). Hormones (Athens). 2016;15(3):394–403.
Meadows KA, Abrams C, Sandbaek A. Adaptation of the Diabetes Health Profile (DHP-1) for use with patients with Type 2 diabetes mellitus: psychometric evaluation and cross-cultural comparison. Diabet Med. 2000;17(8):572–80.
Mulhern B, Meadows K. The construct validity and responsiveness of the EQ-5D, SF-6D and Diabetes Health Profile-18 in type 2 diabetes. Health Qual Life Outcomes. 2014;12:42.
Mulhern B, Labeit A, Rowen D, Knowles E, Meadows K, Elliott J, et al. Developing preference-based measures for diabetes: DHP-3D and DHP-5D. Diabet Med. 2017;34(9):1264–75.
Tan LS, Khoo EY, Tan CS, Griva K, Mohamed A, New M, et al. Sensitivity of three widely used questionnaires for measuring psychological distress among patients with type 2 diabetes mellitus. Qual Life Res. 2015;24(1):153–62.
Benazizi I, Bernal-Soriano MC, Pardo Y, Ribera A, Peralta-Chiriboga A, Ferrer M, et al. Adaptation and psychometric validation of Diabetes Health Profile (DHP-18) in patients with type 2 diabetes in Quito, Ecuador: a cross-sectional study. Health Qual Life Outcomes. 2021;19(1):189.
Goddijn P, Bilo H, Meadows K, Groenier K, Feskens E, Meyboom-de JB. The validity and reliability of the Diabetes Health Profile (DHP) in NIDDM patients referred for insulin therapy. Qual Life Res. 1996;5(4):433–42.
Hammond GS, Aoki TT. Measurement of health status in diabetic patients. Diabetes impact measurement scales Diabetes Care. 1992;15(4):469–77.
Li TC, Lin CC, Liu CS, Li CI, Lee YD. Validation of the Chinese version of the diabetes impact measurement scales amongst people suffering from diabetes. Qual Life Res. 2006;15(10):1613–9.
Lin CY, Lee TY, Sun ZJ, Yang YC, Wu JS, Ou HT. Development of diabetes-specific quality of life module to be in conjunction with the World Health Organization quality of life scale brief version (WHOQOL-BREF). Health Qual Life Outcomes. 2017;15(1):167.
Saffari M, Lin CY, K. OG, Koenig HG, Sanaeinasab H, Pakpour AH. Psychometric properties of Persian Diabetes-Mellitus Specific Quality of Life (DMQoL) questionnaire in a population-based sample of Iranians. Int J Diabet Develop Countries. 2019;39(1):218–27.
Permana H, Liem MV, Soetedjo NNM. Validation of the Indonesian Version of the Asian Diabetes Quality of Life Questionnaire. Acta Med Indones. 2021;53(2):143–8.
Burroughs TE, Desikan R, Waterman BM, Gilin D, McGill J. Development and Validation of the Diabetes Quality of Life Brief Clinical Inventory. Diabetes Spectrum. 2004;17(1):41–9.
Dudzinska M, Tarach JS, Burroughs TE, Zwolak A, Matuszek B, Smolen A, et al. Validation of the Polish version of Diabetes Quality of Life - Brief Clinical Inventory (DQL-BCI) among patients with type 2 diabetes. Arch Med Sci. 2014;10(5):891–8.
Samah S, Neoh CF, Wong YY, Hassali MA, Shafie AA, Lim SM, et al. Linguistic and psychometric validation of the Malaysian version of Diabetes Quality of Life-Brief Clinical Inventory (DQoL-BCI). Res Social Adm Pharm. 2017;13(6):1135–41.
Tang Z, Jiang X, Hong L, Feng Z, He Q, Yuan J, et al. Validation of the Simplified Chinese Version of the Brief Diabetes Quality of Life (DQoL) Questionnaire Based on a Cross-Sectional Study. Int J Environ Res Public Health. 2020;17(23).
Diriba DC, Leung DYP, Suen LKP. Cultural Adaptation and Psychometric Properties of the Diabetes Quality of Life Scale in Afaan Oromoo among People Living with Type 2 Diabetes in Ethiopia. Int J Environ Res Public Health. 2021;18(14).
Cheng AY, Tsui EY, Hanley AJ, Zinman B. Developing a quality of life measure for Chinese patients with diabetes. Diabetes Res Clin Pract. 1999;46(3):259–67.
Cheng AY, Tsui EY, Hanley AJ, Zinman B. Cultural adaptation of the diabetes quality-of-life measure for Chinese patients. Diabetes Care. 1999;22(7):1216–7.
Huang IC, Liu JH, Wu AW, Wu MY, Leite W, Hwang CC. Evaluating the reliability, validity and minimally important difference of the Taiwanese version of the diabetes quality of life (DQOL) measurement. Health Qual Life Outcomes. 2008;6:87.
Yildirim A, Akinci F, Gozu H, Sargin H, Orbay E, Sargin M. Translation, cultural adaptation, cross-validation of the Turkish diabetes quality-of-life (DQOL) measure. Qual Life Res. 2007;16(5):873–9.
Pakpour AH, Saffari M, Burri A. Translation and validation of an Iranian version of the Diabetes Quality of Life measure. J Diabetes Investig. 2012;3(5):471–8.
Sato F, Mita T, Yamamoto R, Hirose T, Ito C, Tamura Y, et al. Reliability and validity of the Japanese version of the Diabetes Quality-Of-Life questionnaire for Japanese patients with type 2 diabetes mellitus. Diabetol Int. 2014;5:21–9.
Rankin SH, Galbraith ME, Huang P. Quality of life and social environment as reported by Chinese immigrants with non-insulin-dependent diabetes mellitus. Diabetes Educ. 1997;23(2):171–7.
Bujang MA, Ismail M, Hatta N, Othman SH, Baharum N, Lazim SSM. Validation of the Malay version of Diabetes Quality of Life (DQOL) Questionnaire for Adult Population with Type 2 Diabetes Mellitus. Malays J Med Sci. 2017;24(4):86–96.
Bujang MA, Adnan TH, Mohd Hatta NKB, Ismail M, Lim CJ. A Revised Version of Diabetes Quality of Life Instrument Maintaining Domains for Satisfaction, Impact, and Worry. J Diabetes Res. 2018;2018:5804687.
Correr CJ, Pontarolo R, Melchiors AC, Rossignoli P, Fernandez-Llimos F, Radominski RB. Translation to portuguese and validation of the Diabetes Quality Of Life Measure (DQOL-Brazil). Arq Bras Endocrinol Metabol. 2008;52(3):515–22.
Brasil F, Brasil AM, e Souza RA, Pontarolo R, Correr CJ. Development of the Brazilian brief version of the Diabetes Quality of Life Measure (DQOL-Brazil-8). Rev Bras Epidemiol. 2015;18(4):943–52.
Jin X, Liu GG, Gerstein HC, Levine MAH, Steeves K, Guan H, et al. Item reduction and validation of the Chinese version of diabetes quality-of-life measure (DQOL). Health Qual Life Outcomes. 2018;16(1):78.
Al-Qerem W, Al-Maayah B, Ling J. Developing and validating the Arabic version of the Diabetes Quality of Life questionnaire. East Mediterr Health J. 2021;27(4):414–26.
Millán MM, Reviriego J, Del Campo J. Reappraisal of the Spanish version of the diabetes quality of life questionnaire (EsDQOL). Endocrinol Nutr. 2002;49(10):322–4.
Alavi NM, Ghofranipour F, Ahmadi F, Emami A. Developing a culturally valid and reliable quality of life questionnaire for diabetes mellitus. East Mediterr Health J. 2007;13(1):177–85.
Jahanlou AS, Karami NA. WHO quality of life-BREF 26 questionnaire: reliability and validity of the Persian version and compare it with Iranian diabetics quality of life questionnaire in diabetic patients. Prim Care Diabetes. 2011;5(2):103–7.
Lee EH, Lee YW, Lee KW, Kim DJ, Kim SK. Development and psychometric evaluation of a diabetes-specific quality-of-life (D-QOL) scale. Diabetes Res Clin Pract. 2012;95(1):76–84.
Grootenhuis PA, Snoek FJ, Heine RJ, Bouter LM. Development of a type 2 diabetes symptom checklist: a measure of symptom severity. Diabet Med. 1994;11(3):253–61.
Arbuckle RA, Humphrey L, Vardeva K, Arondekar B, Danten-Viala M, Scott JA, et al. Psychometric evaluation of the Diabetes Symptom Checklist-Revised (DSC-R)–a measure of symptom distress. Value Health. 2009;12(8):1168–75.
Naegeli AN, Stump TE, Hayes RP. A psychometric evaluation of the Diabetes Symptom Checklist-Revised (DSC-R) cognitive distress, fatigue, hyperglycemia, and hypoglycemia subscales in patients with type 1 and type 2 diabetes. Diabetes Metab Syndr Obes. 2010;3:27–30.
Lee EH, Lee KW, Song R, Snoek FJ, Moon SH. Psychometric evaluation of the Korean version of the Diabetes Symptom Checklist-Revised (DSC-R) for patients with type 2 diabetes. Health Qual Life Outcomes. 2014;12:77.
de Cock ES, Emons WH, Nefs G, Pop VJ, Pouwer F. Dimensionality and scale properties of the Edinburgh Depression Scale (EDS) in patients with type 2 diabetes mellitus: the DiaDDzoB study. BMC Psychiatry. 2011;11:141.
Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Making. 2002;22(4):340–9.
Ekwunife OI, Ezenduka CC, Uzoma BE. Evaluating the sensitivity of EQ-5D in a sample of patients with type 2 diabetes mellitus in two tertiary health care facilities in Nigeria. BMC Res Notes. 2016;9:24.
Konerding U, Elkhuizen SG, Faubel R, Forte P, Malmstrom T, Pavi E, et al. The validity of the EQ-5D-3L items: an investigation with type 2 diabetes patients from six European countries. Health Qual Life Outcomes. 2014;12:181.
Lee WJ, Song KH, Noh JH, Choi YJ, Jo MW. Health-related quality of life using the EuroQol 5D questionnaire in Korean patients with type 2 diabetes. J Korean Med Sci. 2012;27(3):255–60.
Luo N, Cang SQ, Quah HM, How CH, Tay EG. The discriminative power of the EuroQol visual analog scale is sensitive to survey language in Singapore. Health Qual Life Outcomes. 2012;10:32.
Matza LS, Boye KS, Yurgin N. Validation of two generic patient-reported outcome measures in patients with type 2 diabetes. Health Qual Life Outcomes. 2007;5:47.
Pan CW, Sun HP, Wang X, Ma Q, Xu Y, Luo N, et al. The EQ-5D-5L index score is more discriminative than the EQ-5D-3L index score in diabetes patients. Qual Life Res. 2015;24(7):1767–74.
Pattanaphesaj J, Thavorncharoensap M. Measurement properties of the EQ-5D-5L compared to EQ-5D-3L in the Thai diabetes patients. Health Qual Life Outcomes. 2015;13:14.
Wang P, Luo N, Tai ES, Thumboo J. The EQ-5D-5L is More Discriminative Than the EQ-5D-3L in Patients with Diabetes in Singapore. Value Health Reg Issues. 2016;9:57–62.
Arifin B, Purba FD, Herman H, Adam JMF, Atthobari J, Schuiling-Veninga CCM, et al. Comparing the EQ-5D-3 L and EQ-5D-5 L: studying measurement and scores in Indonesian type 2 diabetes mellitus patients. Health Qual Life Outcomes. 2020;18(1):22.
Zare F, Ameri H, Madadizadeh F, Aghaei MR. Validity and reliability of the EQ-5D-3L (a generic preference-based instrument used for calculating quality-adjusted life -years) for patients with type 2 diabetes in Iran. Diabetes Metab Syndr. 2021;15(1):319–24.
Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res. 2013;22(7):1717–27.
Koh D, Abdullah AM, Wang P, Lin N, Luo N. Validation of Brunei’s Malay EQ-5D Questionnaire in Patients with Type 2 Diabetes. PLoS ONE. 2016;11(11): e0165555.
Matza LS, Boye KS, Stewart KD, Curtis BH, Reaney M, Landrian AS. A qualitative examination of the content validity of the EQ-5D-5L in patients with type 2 diabetes. Health Qual Life Outcomes. 2015;13:192.
Sayah FA, Qiu W, Xie F, Johnson JA. Comparative performance of the EQ-5D-5L and SF-6D index scores in adults with type 2 diabetes. Qual Life Res. 2017;26(8):2057–66.
Wang Y, Tan NC, Tay EG, Thumboo J, Luo N. Cross-cultural measurement equivalence of the 5-level EQ-5D (EQ-5D-5L) in patients with type 2 diabetes mellitus in Singapore. Health Qual Life Outcomes. 2015;13:103.
Cinar D, Yava A. Validity and reliability of functional assessment of chronic illness treatment-fatigue scale in Turkish patients with type 2 diabetes. Endocrinol Diabetes Nutr. 2018;65(7):409–17.
Leonardson GR, Daniels MC, Ness FK, Kemper E, Mihura JL, Koplin BA, et al. Validity and reliability of the general well-being schedule with northern plains American Indians diagnosed with type 2 diabetes mellitus. Psychol Rep. 2003;93(1):49–58.
Amidu N, Alhassan A, Owiredu W, Alidu H, Antuamwine BB, Abdul-Wahid M. Validity and Reliability of the Golombok-Rust Inventory of Sexual Satisfaction (GRISS) in Patients With Type 2 Diabetes. J Sex Marital Ther. 2019;45(2):141–7.
Hajos TR, Polonsky WH, Pouwer F, Gonder-Frederick L, Snoek FJ. Toward defining a cutoff score for elevated fear of hypoglycemia on the hypoglycemia fear survey worry subscale in patients with type 2 diabetes. Diabetes Care. 2014;37(1):102–8.
Kawata AK, Wilson H, Ong SH, Kulich K, Coyne K. Development and Psychometric Evaluation of the Hypoglycemia Perspectives Questionnaire in Patients with Type 2 Diabetes Mellitus. Patient. 2016;9(5):395–407.
Maddigan SL, Feeny DH, Majumdar SR, Farris KB, Johnson JA. Health Utilities Index mark 3 demonstrated construct validity in a population-based sample with type 2 diabetes. J Clin Epidemiol. 2006;59(5):472–7.
Mo F, Morrison H, Choi BC, Vardy L. Evaluation and measurement of health-related quality of life for individuals with diabetes mellitus by Health Utilities Index Mark 3 (HUI3) system. Scie World J. 2006;6:1412–23.
Kolotkin RL, Crosby RD, Williams GR. Assessing weight-related quality of life in obese persons with type 2 diabetes. Diabetes Res Clin Pract. 2003;61(2):125–32.
Kolotkin RL, Ervin CM, Meincke HH, Hojbjerre L, Fehnel SE. Development of a clinical trials version of the Impact of Weight on Quality of Life-Lite questionnaire (IWQOL-Lite Clinical Trials Version): results from two qualitative studies. Clin Obes. 2017;7(5):290–9.
Kolotkin RL, Williams VSL, Ervin CM, Williams N, Meincke HH, Qin S, et al. Validation of a new measure of quality of life in obesity trials: Impact of Weight on Quality of Life-Lite Clinical Trials Version. Clin Obes. 2019;9(3): e12310.
Hasan SS, Ahmadi K, Santigo R, Ahmed SI. The validity of the Menopause-specific Quality of Life questionnaire in women with type 2 diabetes. Climacteric. 2014;17(4):456–64.
Kim SS, Won JC, Kwon HS, Kim CH, Lee JH, Park TS, et al. Validity of the medical outcomes study sleep scale in patients with painful diabetic peripheral neuropathy in Korea. J Diabetes Investig. 2013;4(4):405–9.
Borg S, Eeg-Olofsson K, Palaszewski B, Svedbo Engstrom M, Gerdtham UG, Gudbjornsdottir S. Patient-reported outcome and experience measures for diabetes: development of scale models, differences between patient groups and relationships with cardiovascular and diabetes complication risk factors, in a combined registry and survey study in Sweden. BMJ Open. 2019;9(1): e025033.
Svedbo Engstrom M, Leksell J, Johansson UB, Eeg-Olofsson K, Borg S, Palaszewski B, et al. A disease-specific questionnaire for measuring patient-reported outcomes and experiences in the Swedish National Diabetes Register: Development and evaluation of content validity, face validity, and test-retest reliability. Patient Educ Couns. 2018;101(1):139–46.
Svedbo Engstrom M, Leksell J, Johansson UB, Borg S, Palaszewski B, Franzen S, et al. New Diabetes Questionnaire to add patients’ perspectives to diabetes care for adults with type 1 and type 2 diabetes: nationwide cross-sectional study of construct validity assessing associations with generic health-related quality of life and clinical variables. BMJ Open. 2020;10(11): e038966.
Vileikyte L, Peyrot M, Bundy C, Rubin RR, Leventhal H, Mora P, et al. The development and validation of a neuropathy- and foot ulcer-specific quality of life instrument. Diabetes Care. 2003;26(9):2549–55.
Keinanen-Kiukaanniemi S, Ohinmaa A, Pajunpaa H, Koivukangas P. Health related quality of life in diabetic patients measured by the Nottingham Health Profile. Diabet Med. 1996;13(4):382–8.
McGuire BE, Morrison TG, Hermanns N, Skovlund S, Eldrup E, Gagliardino J, et al. Short-form measures of diabetes-related emotional distress: the Problem Areas in Diabetes Scale (PAID)-5 and PAID-1. Diabetologia. 2010;53(1):66–9.
Lee EH, Lee YW, Lee KW, Kim YS, Nam MS. Measurement of diabetes-related emotional distress using the Problem Areas in Diabetes scale: psychometric evaluations show that the short form is better than the full form. Health Qual Life Outcomes. 2014;12:142.
Vislapuu M, Brostrom A, Igland J, Vorderstrasse A, Iversen MM. Psychometric properties of the Norwegian version of the short form of The Problem Areas in Diabetes scale (PAID-5): a validation study. BMJ Open. 2019;9(2): e022903.
Siaw MY, Tai BB, Lee JY. Psychometric properties of the Chinese version of the Problem Areas in Diabetes scale (SG-PAID-C) among high-risk polypharmacy patients with uncontrolled type 2 diabetes in Singapore. J Diabetes Investig. 2017;8(2):235–42.
Venkataraman K, Tan LS, Bautista DC, Griva K, Zuniga YL, Amir M, et al. Psychometric Properties of the Problem Areas in Diabetes (PAID) Instrument in Singapore. PLoS ONE. 2015;10(9): e0136759.
Arzaghi sm, Mahjouri M, Heshmat R, Khashayar P, Larijani B. Psychometric properties of the Iranian version of the Problem Areas in Diabetes scale (IR-PAID-20). Iran J Diabetes Metab Disord. 2010;10.
Belendez M, Hernandez-Mijares A, Marco J, Dominguez JR, Pomares FJ. Validation of the Spanish version of the Problem Areas in Diabetes (PAID-SP) Scale. Diabetes Res Clin Pract. 2014;106(3):e93–5.
Eom YS, Park HS, Kim SH, Yang SM, Nam MS, Lee HW, et al. Evaluation of stress in korean patients with diabetes mellitus using the problem areas in diabetes-Korea questionnaire. Diabetes Metab J. 2011;35(2):182–7.
Gross CC, Scain SF, Scheffel R, Gross JL, Hutz CS. Brazilian version of the Problem Areas in Diabetes Scale (B-PAID): validation and identification of individuals at high risk for emotional distress. Diabetes Res Clin Pract. 2007;76(3):455–9.
Huang MF, Courtney M, Edwards H, McDowell J. Validation of the Chinese version of the Problem Areas in Diabetes (PAID-C) scale. Diabetes Care. 2010;33(1):38–40.
Huis In 't Veld EM, Makine C, Nouwen A, Karsidag C, Kadioglu P, Karsidag K, et al. Validation of the Turkish version of the problem areas in diabetes scale. Cardiovasc Psychiatry Neurol. 2011;2011:315068.
Jannoo Z, Yap BW, Khan NM, Farcomeni A. Assessing Diabetes Distress Among Type 2 Diabetes Mellitus in Malaysia Using the Problem Areas in Diabetes Scale. Value in Health Regional Issues. 2019;18:159–64.
Miller ST, Elasy TA. Psychometric evaluation of the Problem Areas in Diabetes (PAID) survey in Southern, rural African American women with Type 2 diabetes. BMC Public Health. 2008;8:70.
Papathanasiou A, Koutsovasilis A, Shea S, Philalithis A, Papavasiliou S, Melidonis A, et al. The Problem Areas in Diabetes (PAID) scale: psychometric evaluation survey in a Greek sample with type 2 diabetes. J Psychiatr Ment Health Nurs. 2014;21(4):345–53.
Polonsky WH, Anderson BJ, Lohrer PA, Welch G, Jacobson AM, Aponte JE, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18(6):754–60.
Snoek FJ, Pouwer F, Welch GW, Polonsky WH. Diabetes-related emotional distress in Dutch and U.S. diabetic patients: cross-cultural validity of the problem areas in diabetes scale. Diabetes Care. 2000;23(9):1305–9.
Welch GW, Jacobson AM, Polonsky WH. The Problem Areas in Diabetes Scale. An evaluation of its clinical utility. Diabetes Care. 1997;20(5):760–6.
Welch G, Weinger K, Anderson B, Polonsky WH. Responsiveness of the Problem Areas In Diabetes (PAID) questionnaire. Diabet Med. 2003;20(1):69–72.
Welch G, Schwartz CE, Santiago-Kelly P, Garb J, Shayne R, Bode R. Disease-related emotional distress of Hispanic and non-Hispanic type 2 diabetes patients. Ethn Dis. 2007;17(3):541–7.
Cichon E, Kiejna A, Gondek TM, Obrebski M, Sutkowska E, Lloyd CE, et al. PAID-PL-The Polish Version of the Problem Areas in Diabetes Scale: Perfect Reliability and a One-Factor Structure. Diabetes Metab Syndr Obes. 2021;14:4433–41.
Zhu B, Xie M, Park CG, Kapella MC. Adaptation of the Pittsburgh Sleep Quality Index in Chinese adults with type 2 diabetes. J Chin Med Assoc. 2018;81(3):242–7.
Orozco-Beltran D, Artola S, Jansa M, Lopez de la Torre-Casares M, Fuster E. Impact of hypoglycemic episodes on health-related quality of life of type-2 diabetes mellitus patients: development and validation of a specific QoLHYPO((c)) questionnaire. Health Qual Life Outcomes. 2018;16(1):52.
Mikhael EM, Hassali MA, Hussain SA, Shawky N. The Development and Validation of Quality of Life Scale for Iraqi Patients with Type 2 Diabetes Mellitus. J Pharm Bioallied Sci. 2020;12(3):262–8.
Whitty P, Steen N, Eccles M, McColl E, Hewison J, Meadows K, et al. A new self-completion outcome measure for diabetes: is it responsive to change? Qual Life Res. 1997;6(5):407–13.
Hsu HC, Chang YH, Lee PJ, Chen SY, Hsieh CH, Lee YJ, et al. Developing and psychometric testing of a short-form problem areas in diabetes scale in chinese patients. J Nurs Res. 2013;21(3):212–8.
Wicaksana AL, Pramono RB, Irianti SR, Khusna RP, Rahayu FP, Aini FN, et al. Screening for psychological distress on Indonesian type 2 diabetes: A validation study. Int J Nurs Pract. 2021;27(6): e12999.
Lupascu N, Timar B, Albai A, Roman D, Potre O, Timar R. Validation and cross-cultural adaptation of the depression Patient’s Health Questionnaire - 9 in the Romanian population of patients with Type 2 Diabetes Mellitus. Diabetes Metab Syndr Obes. 2019;12:841–9.
Twist K, Stahl D, Amiel SA, Thomas S, Winkley K, Ismail K. Comparison of depressive symptoms in type 2 diabetes using a two-stage survey design. Psychosom Med. 2013;75(8):791–7.
Udedi M, Muula AS, Stewart RC, Pence BW. The validity of the patient health Questionnaire-9 to screen for depression in patients with type-2 diabetes mellitus in non-communicable diseases clinics in Malawi. BMC Psychiatry. 2019;19(1):81.
Zhang Y, Ting R, Lam M, Lam J, Nan H, Yeung R, et al. Measuring depressive symptoms using the Patient Health Questionnaire-9 in Hong Kong Chinese subjects with type 2 diabetes. J Affect Disord. 2013;151(2):660–6.
Duran G, Herschbach P, Waadt S, Strian F, Zettler A. Assessing daily problems with diabetes: a subject-oriented approach to compliance. Psychol Rep. 1995;76(2):515–21.
Herschbach P, Duran G, Waadt S, Zettler A, Amm C, Marten-Mittag B. Psychometric properties of the Questionnaire on Stress in Patients with Diabetes-Revised (QSD-R). Health Psychol. 1997;16(2):171–4.
Kinik Çamlicali Ç. Reliability and validity of the questionnaire on stress in patients with diabetes-revised (QSD-R): The Turkish adaptation (QSD-R-TR): Fatih University; 2016.
Fraim NL. The Turkish Adaptation of the QSD-R in the Turkish Republic of Northern Cyprus. J Psychol Clin Psychiatry. 2015;4(3):00219.
Pakpour AH, Zeidi IM, Saffari M, Burri A. Psychometric properties of the Iranian version of the Sexual Quality of Life Scale among women. J Sex Med. 2013;10(4):981–9.
Polonsky WH, Fisher L, Hessler D, Desai U, King SB, Perez-Nieves M. Toward a more comprehensive understanding of the emotional side of type 2 diabetes: A re-envisioning of the assessment of diabetes distress. J Diabetes Complications. 2022;36(1): 108103.
Pouwer F, van der Ploeg HM, Ader HJ, Heine RJ, Snoek FJ. The 12-item well-being questionnaire. An evaluation of its validity and reliability in Dutch people with diabetes. Diabetes Care. 1999;22(12):2004–10.
Pouwer F, Snoek FJ, van der Ploeg HM, Ader HJ, Heine RJ. The well-being questionnaire: evidence for a three-factor structure with 12 items (W-BQ12). Psychol Med. 2000;30(2):455–62.
Kolawole BA, Abodunde O, Ikem RT, Fabiyi AK. A test of the reliability and validity of a diabetes specific quality of life scale in a Nigerian hospital. Qual Life Res. 2004;13(7):1287–95.
Speight J, Khagram LA, Davies MJ. Generic and diabetes-specific well-being in the AT.LANTUS Follow-on study: further psychometric validation of the W-BQ28 indicates its utility in research and clinical practice in Type 2 diabetes in the UK. Diabet Med. 2012;29(9):e345–53.
Bradley C, Lewis KS. Measures of psychological well-being and treatment satisfaction developed from the responses of people with tablet-treated diabetes. Diabet Med. 1990;7(5):445–51.
Awata S, Bech P, Yoshida S, Hirai M, Suzuki S, Yamashita M, et al. Reliability and validity of the Japanese version of the World Health Organization-Five Well-Being Index in the context of detecting depression in diabetic patients. Psychiatry Clin Neurosci. 2007;61(1):112–9.
Cichoń E, Kiejna A, Kokoszka A, Gondek T, Rajba B, Lloyd CE, et al. Validation of the Polish version of WHO-5 as a screening instrument for depression in adults with diabetes. Diabetes Res Clin Pract. 2020;159: 107970.
Hajos TR, Pouwer F, Skovlund SE, Den Oudsten BL, Geelhoed-Duijvestijn PH, Tack CJ, et al. Psychometric and screening properties of the WHO-5 well-being index in adult outpatients with Type 1 or Type 2 diabetes mellitus. Diabet Med. 2013;30(2):e63–9.
Halliday JA, Hendrieckx C, Busija L, Browne JL, Nefs G, Pouwer F, et al. Validation of the WHO-5 as a first-step screening instrument for depression in adults with diabetes: Results from Diabetes MILES - Australia. Diabetes Res Clin Pract. 2017;132:27–35.
Reba K, Birhane BW, Gutema H. Validity and Reliability of the Amharic Version of the World Health Organization’s Quality of Life Questionnaire (WHOQOL-BREF) in Patients with Diagnosed Type 2 Diabetes in Felege Hiwot Referral Hospital. Ethiopia J Diabetes Res. 2019;2019:3513159.
Nguyen H, Butow P, Dhillon H, Sundaresan P. A review of the barriers to using Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs) in routine cancer care. J Med Radiat Sci. 2021;68(2):186–95.
Terwee CB, Elders PJM, Langendoen-Gort M, Elsman EBM, Prinsen CAC, van der Heijden AA, et al. Content validity of patient-reported outcome measures developed for assessing health-related quality of life in people with type 2 diabetes mellitus - a systematic review. Accepted for publication in Current Diabetes Reports. 2022.
Dodd S, Harman N, Taske N, Minchin M, Tan T, Williamson PR. Core outcome sets through the healthcare ecosystem: the case of type 2 diabetes mellitus. Trials. 2020;21(1):570.
Harman NL, Wilding JPH, Curry D, Harris J, Logue J, Pemberton RJ, et al. Selecting Core Outcomes for Randomised Effectiveness trials In Type 2 diabetes (SCORE-IT): a patient and healthcare professional consensus on a core outcome set for type 2 diabetes. BMJ Open Diabetes Res Care. 2019;7(1): e000700.
ICHOM. Standard set for Diabetes 2019 [Available from: https://connect.ichom.org/standard-sets/diabetes/.
Svedbo Engstrom M, Leksell J, Johansson UB, Gudbjornsdottir S. What is important for you? A qualitative interview study of living with diabetes and experiences of diabetes care to establish a basis for a tailored Patient-Reported Outcome Measure for the Swedish National Diabetes Register. BMJ Open. 2016;6(3): e010249.
Funding
F. Rutters is funded through an EFSD award supported by EFSD/Sanofi European Diabetes Research Programme in Macrovascular Complications.
Author information
Authors and Affiliations
Contributions
All authors have contributed in a meaningful way. CBT and CACP had the original idea, and CBT completed the searches. JWB, PJME, AAH, IH, MLG, GM, CACP, FR, CBT and MW conducted the screening for relevant papers and LG, MLG, FR and CBT extracted the data. MLG, LG, FR and CBT conducted the analyses and made the first draft of the manuscript. All authors (JWB, PJME, IH, LG, MLG, GM, CACP, FR and CBT) have commented on the manuscript and likewise, all authors have read and approved the final manuscript. FR and CBT are the guarantors of this work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Research in context
What is already known about this subject?
- Patient-Reported Outcome Measures (PROMs) are important tools to assess outcomes relevant to patients, with Health-Related Quality Of Life (HRQOL) as an important construct to be measured.
- Many different HRQOL PROMs are used in the type 2 diabetes field, however a complete overview of these PROMs is currently lacking.
What is the key question?
- Can we systematically describe and classify the content of all PROMs that have specifically been developed or validated to measure (aspects of) HRQOL in people with type 2 diabetes?
What are the new findings?
- Of the 116 unique HRQOL PROMs, 91 (of the subscales) measured symptom status, 60 measured functional status and 26 measured general health perceptions.
- About half of the PROMs (subscales) also include characteristics of the individual or the environment or patient-reported experience measures (PREMs), which are not part of the HRQOL construct.
- About 5% of the PROMs only measured global quality of life and/or PREMs and thus no HRQOL construct.
How might this impact on clinical practice in the near future?
- There is a need for consensus on which aspects of HRQOL should be measured in people with type 2 diabetes and which PROMs to use in research and daily practice.
Supplementary information
Below is the link to the electronic supplementary material.
Appendix 1 Search strategy
Appendix 1 Search strategy
1.1 PubMed search April 29, 2019
1.1.1 #1 Diabetes type 2
((Diabet*[tiab] AND (("non insulin"[tiab] AND depend*[tiab]) OR ("noninsulin"[tiab] AND depend*[tiab]) OR “type 2”[tiab] OR “type II” [tiab])) OR iddm[tiab] OR niddm[tiab] OR “glucose intolerance”[tiab] OR “insulin resistant”[tiab] OR “insulin resistance”[tiab]).
1.1.2 #2 Modified filter for studies on measurement properties*
"Validation Studies"[pt] OR "psychometrics"[MeSH] OR psychometr*[tiab] OR clinimetr*[tw] OR clinometr*[tw] OR "outcome assessment (health care)"[MeSH] OR "outcome assessment"[tiab] OR "outcome measure*"[tw] OR "observer variation"[MeSH] OR "observer variation"[tiab] OR "reproducibility of results"[MeSH] OR reproducib*[tiab] OR "discriminant analysis"[MeSH] OR reliab*[tiab] OR unreliab*[tiab] OR valid*[tiab] OR "coefficient of variation"[tiab] OR homogeneity[tiab] OR homogeneous[tiab] OR "internal consistency"[tiab] OR (cronbach*[tiab] AND (alpha[tiab] OR alphas[tiab])) OR (item[tiab] AND (correlation*[tiab] OR selection*[tiab] OR reduction*[tiab])) OR agreement[tw] OR precision[tw] OR imprecision[tw] OR "precise values"[tw] OR test–retest[tiab] OR (test[tiab] AND retest[tiab]) OR (reliab*[tiab] AND (test[tiab] OR retest[tiab])) OR stability[tiab] OR interrater[tiab] OR inter-rater[tiab] OR intrarater[tiab] OR intra-rater[tiab] OR intertester[tiab] OR inter-tester[tiab] OR intratester[tiab] OR intra-tester[tiab] OR interobserver[tiab] OR inter-observer[tiab] OR intraobserver[tiab] OR intra-observer[tiab] OR intertechnician[tiab] OR inter-technician[tiab] OR intratechnician[tiab] OR intra-technician[tiab] OR interexaminer[tiab] OR inter-examiner[tiab] OR intraexaminer[tiab] OR intra-examiner[tiab] OR interassay[tiab] OR inter-assay[tiab] OR intraassay[tiab] OR intra-assay[tiab] OR interindividual[tiab] OR inter-individual[tiab] OR intraindividual[tiab] OR intra-individual[tiab] OR interparticipant[tiab] OR inter-participant[tiab] OR intraparticipant[tiab] OR intra-participant[tiab] OR kappa[tiab] OR kappa's[tiab] OR kappas[tiab] OR repeatab*[tw] OR ((replicab*[tw] OR repeated[tw]) AND (measure[tw] OR measures[tw] OR findings[tw] OR test[tw] OR tests[tw])) OR generaliza*[tiab] OR generalisa*[tiab] OR concordance[tiab] OR (intraclass[tiab] AND correlation*[tiab]) OR discriminative[tiab] OR "known group"[tiab] OR "factor analysis"[tiab] OR "factor analyses"[tiab] OR "factor structure"[tiab] OR "factor structures"[tiab] OR subscale*[tiab] OR (multitrait[tiab] AND scaling[tiab] AND (analysis[tiab] OR analyses[tiab])) OR "item discriminant"[tiab] OR "interscale correlation*"[tiab] OR error[tiab] OR errors[tiab] OR "individual variability"[tiab] OR "interval variability"[tiab] OR "rate variability"[tiab] OR (variability[tiab] AND (analysis[tiab] OR values[tiab])) OR "standard error of measurement"[tiab] OR responsive*[tiab] OR (limit[tiab] AND detection[tiab]) OR "minimal detectable concentration"[tiab] OR interpretab*[tiab] OR ((minimal[tiab] OR minimally[tiab] OR clinical[tiab] OR clinically[tiab]) AND (important[tiab] OR detectable[tiab]) AND (change[tiab] OR difference[tiab])) OR (small*[tiab] AND (real[tiab] OR detectable[tiab]) AND (change[tiab] OR difference[tiab])) OR "meaningful change"[tiab] OR "ceiling effect"[tiab] OR "floor effect"[tiab] OR "Item response model"[tiab] OR IRT[tiab] OR Rasch[tiab] OR "Differential item functioning"[tiab] OR DIF[tiab] OR "computer adaptive testing"[tiab] OR "item bank"[tiab] OR "cross-cultural equivalence"[tiab].
1.1.3 #3 PROM filter (developed by the University of Oxford, see www.comin.nl)
(HR-PRO[tiab] OR HRPRO[tiab] OR HRQL[tiab] OR HRQoL[tiab] OR QL[tiab] OR QoL[tiab] OR quality of life[tw] OR life quality[tw] OR health index*[tiab] OR health indices[tiab] OR health profile*[tiab] OR health status[tw] OR ((patient[tiab] OR self[tiab] OR child[tiab] OR parent[tiab] OR carer[tiab] OR proxy[tiab]) AND ((report[tiab] OR reported[tiab] OR reporting[tiab]) OR (rated[tiab] OR rating[tiab] OR ratings[tiab]) OR based[tiab] OR (assessed[tiab] OR assessment[tiab] OR assessments[tiab]))) OR ((disability[tiab] OR function[tiab] OR functional[tiab] OR functions[tiab] OR subjective[tiab] OR utility[tiab] OR utilities[tiab] OR wellbeing[tiab] OR well being[tiab]) AND (index[tiab] OR indices[tiab] OR instrument[tiab] OR instruments[tiab] OR measure[tiab] OR measures[tiab] OR questionnaire[tiab] OR questionnaires[tiab] OR profile[tiab] OR profiles[tiab] OR scale[tiab] OR scales[tiab] OR score[tiab] OR scores[tiab] OR status[tiab] OR survey[tiab] OR surveys[tiab]))).
(#1 AND #2 AND #3) NOT ("addresses"[Publication Type] OR "biography"[Publication Type] OR "case reports"[Publication Type] OR "comment"[Publication Type] OR "directory"[Publication Type] OR "editorial"[Publication Type] OR "festschrift"[Publication Type] OR "interview"[Publication Type] OR "lectures"[Publication Type] OR "legal cases"[Publication Type] OR "legislation"[Publication Type] OR "letter"[Publication Type] OR "news"[Publication Type] OR "newspaper article"[Publication Type] OR "patient education handout"[Publication Type] OR "popular works"[Publication Type] OR "congresses"[Publication Type] OR "consensus development conference"[Publication Type] OR "consensus development conference, nih"[Publication Type] OR "practice guideline"[Publication Type]) NOT ("animals"[MeSH Terms] NOT "humans"[MeSH Terms]).
1.2 EMBASE search April 29, 2019
1.2.1 #1 Diabetes type 2
(Diabet*:ti,ab AND (('non insulin':ti,ab AND depend*:ti,ab) OR (noninsulin:ti,ab AND depend*:ti,ab) OR 'type 2':ti,ab OR 'type II':ti,ab)) OR iddm:ti,ab OR niddm:ti,ab OR 'glucose intolerance':ti,ab OR 'insulin resistant':ti,ab OR 'insulin resistance':ti,ab.
1.2.2 #2 Modified filter for studies on measurement properties*
'data collection method'/exp OR 'validation study'/exp OR 'feasibility study'/exp OR 'pilot study'/exp OR 'psychometry'/exp OR 'reproducibility'/exp OR reproducib*:ab,ti OR 'audit':ab,ti OR psychometr*:ab,ti OR clinimetr*:ab,ti OR clinometr*:ab,ti OR 'observer variation'/exp OR 'observer variation':ab,ti OR 'discriminant analysis'/exp OR 'validity'/exp OR reliab*:ab,ti OR valid*:ab,ti OR 'internal consistency':ab,ti OR (cronbach*:ab,ti AND ('alpha':ab,ti OR 'alphas':ab,ti)) OR 'item correlation':ab,ti OR 'item correlations':ab,ti OR 'item selection':ab,ti OR 'item selections':ab,ti OR 'item reduction':ab,ti OR 'item reductions':ab,ti OR 'agreement':ab,ti OR 'precision':ab,ti OR 'imprecision':ab,ti OR 'precise values':ab,ti OR 'test–retest':ab,ti OR ('test':ab,ti AND 'retest':ab,ti) OR (reliab*:ab,ti AND ('test':ab,ti OR 'retest':ab,ti)) OR 'stability':ab,ti OR 'interrater':ab,ti OR 'inter-rater':ab,ti OR 'intrarater':ab,ti OR 'intra-rater':ab,ti OR 'intertester':ab,ti OR 'inter-tester':ab,ti OR 'intratester':ab,ti OR 'intra-tester':ab,ti OR 'interobeserver':ab,ti OR 'inter-observer':ab,ti OR 'intraobserver':ab,ti OR 'intra-observer':ab,ti OR 'intertechnician':ab,ti OR 'inter-technician':ab,ti OR 'intratechnician':ab,ti OR 'intra-technician':ab,ti OR 'interexaminer':ab,ti OR 'inter-examiner':ab,ti OR 'intraexaminer':ab,ti OR 'intra-examiner':ab,ti OR 'interassay':ab,ti OR 'inter-assay':ab,ti OR 'intraassay':ab,ti OR 'intra-assay':ab,ti OR 'interindividual':ab,ti OR 'inter-individual':ab,ti OR 'intraindividual':ab,ti OR 'intra-individual':ab,ti OR 'interparticipant':ab,ti OR 'inter-participant':ab,ti OR 'intraparticipant':ab,ti OR 'intra-participant':ab,ti OR 'kappa':ab,ti OR 'kappas':ab,ti OR 'coefficient of variation':ab,ti OR repeatab*:ab,ti OR (replicab*:ab,ti OR 'repeated':ab,ti AND ('measure':ab,ti OR 'measures':ab,ti OR 'findings':ab,ti OR 'test':ab,ti OR 'tests':ab,ti)) OR generaliza*:ab,ti OR generalisa*:ab,ti OR 'concordance':ab,ti OR ('intraclass':ab,ti AND correlation*:ab,ti) OR 'discriminative':ab,ti OR 'known group':ab,ti OR 'factor analysis':ab,ti OR 'factor analyses':ab,ti OR 'factor structure':ab,ti OR 'factor structures':ab,ti OR 'dimensionality':ab,ti OR subscale*:ab,ti OR 'multitrait scaling analysis':ab,ti OR 'multitrait scaling analyses':ab,ti OR 'item discriminant':ab,ti OR 'interscale correlation':ab,ti OR 'interscale correlations':ab,ti OR ('error':ab,ti OR 'errors':ab,ti AND (measure*:ab,ti OR correlat*:ab,ti OR evaluat*:ab,ti OR 'accuracy':ab,ti OR 'accurate':ab,ti OR 'precision':ab,ti OR 'mean':ab,ti)) OR 'individual variability':ab,ti OR 'interval variability':ab,ti OR 'rate variability':ab,ti OR 'variability analysis':ab,ti OR 'standard error of measurement':ab,ti OR responsive*:ab,ti OR ('limit':ab,ti AND 'detection':ab,ti) OR 'minimal detectable concentration':ab,ti OR interpretab*:ab,ti OR (small*:ab,ti AND ('real':ab,ti OR 'detectable':ab,ti) AND ('change':ab,ti OR 'difference':ab,ti)) OR 'meaningful change':ab,ti OR 'minimal important change':ab,ti OR 'minimal important difference':ab,ti OR 'minimally important change':ab,ti OR 'minimally important difference':ab,ti OR 'minimal detectable change':ab,ti OR 'minimal detectable difference':ab,ti OR 'minimally detectable change':ab,ti OR 'minimally detectable difference':ab,ti OR 'minimal real change':ab,ti OR 'minimal real difference':ab,ti OR 'minimally real change':ab,ti OR 'minimally real difference':ab,ti OR 'ceiling effect':ab,ti OR 'floor effect':ab,ti OR 'item response model':ab,ti OR 'irt':ab,ti OR 'rasch':ab,ti OR 'differential item functioning':ab,ti OR 'dif':ab,ti OR 'computer adaptive testing':ab,ti OR 'item bank':ab,ti OR 'cross-cultural equivalence':ab,ti.
1.2.3 #3 PROM filter (developed by the University of Oxford, see www.comin.nl)
(HR-PRO:ti,ab OR HRPRO:ti,ab OR HRQL:ti,ab OR HRQoL:ti,ab OR QL:ti,ab OR QoL:ti,ab OR 'quality of life':ti,ab OR 'life quality':ti,ab OR 'health index*':ti,ab OR 'health indices':ti,ab OR 'health profile*':ti,ab OR 'health status':ti,ab OR ((patient:ti,ab OR self:ti,ab OR child:ti,ab OR parent:ti,ab OR carer:ti,ab OR proxy:ti,ab) AND ((report:ti,ab OR reported:ti,ab OR reporting:ti,ab) OR (rated:ti,ab OR rating:ti,ab OR ratings:ti,ab) OR based:ti,ab OR (assessed:ti,ab OR assessment:ti,ab OR assessments:ti,ab))) OR ((disability:ti,ab OR function:ti,ab OR functional:ti,ab OR functions:ti,ab OR subjective:ti,ab OR utility:ti,ab OR utilities:ti,ab OR wellbeing:ti,ab OR 'well being':ti,ab) AND (index:ti,ab OR indices:ti,ab OR instrument:ti,ab OR instruments:ti,ab OR measure:ti,ab OR measures:ti,ab OR questionnaire:ti,ab OR questionnaires:ti,ab OR profile:ti,ab OR profiles:ti,ab OR scale:ti,ab OR scales:ti,ab OR score:ti,ab OR scores:ti,ab OR status:ti,ab OR survey:ti,ab OR surveys:ti,ab))).
1.2.4 #4 publicatie types
#3 AND ('article'/it OR 'article in press'/it OR 'review'/it).
1.2.5 #5 not animals
#4 NOT ([animals]/lim NOT [humans]/lim).
* Modified from Terwee et al. (19): a few search terms were left out to decrease the number of abstracts needed to be read.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Langendoen-Gort, M., Groeneveld, L., Prinsen, C.A.C. et al. Patient-reported outcome measures for assessing health-related quality of life in people with type 2 diabetes: A systematic review. Rev Endocr Metab Disord 23, 931–977 (2022). https://doi.org/10.1007/s11154-022-09734-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-022-09734-9