Abstract
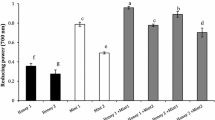
Several monofloral Cuban honeys were analyzed to determine their free radical-scavenging activity and from this the total antioxidant content was estimated. The protective effect against lipid peroxidation in an in vitro model of rat liver homogenates was evaluated and, lastly, the mineral content of the honeys, which can be related to the maintenance of intracellular oxidative balance, was determined. The scavenging capacities against hydroxyl and superoxide radicals were determined using the spin-trapping technique and the hypoxanthine/xanthine oxidase assay, respectively. Lipid peroxidation was evaluated through the production of TBARS and hydroperoxides. All honeys tested showed potential antioxidant activity with Linen vine displaying the highest scavenging capacity towards the DPPH, hydroxyl and superoxide radicals, while the least efficient was Christmas vine honey. Honeys also inhibited, in a concentration-dependent mode, lipid peroxidation in rat liver homogenates, with Linen vine resulting the best while the least effective was Christmas vine honey. The ability to scavenge free radicals and protect against lipid peroxidation may contribute to the ability of certain Cuban honeys to help in preventing/reducing some inflammatory diseases in which oxidative stress is involved. A total of eight minerals were identified and quantified as follows: cadmium, chromium, copper, nickel, iron, manganese, lead, and zinc. Minerals found in higher concentrations were iron, zinc and manganese.

Similar content being viewed by others
Abbreviations
- AA:
-
Antioxidant Activity
- AEAC:
-
Ascorbic Acid Equivalent Antioxidant Content
- EPR:
-
Electron Paramagnetic Resonance Spectroscopy
- MCH:
-
Monofloral Cuban Honey
- QEAC:
-
Quercetin Equivalent Antioxidant Content
- RSA:
-
Radical Scavenging Activity
- TBARS:
-
Thiobarbituric Reactive Substances
- RLH:
-
Rat Liver Homogenate
References
Alvarez-Suarez JM, Tulipani S, Romandini S, Bertoli E, Battino M (2010) Contribution of honey in nutrition and human health: A review. Mediterr J Nutr Metab 3:15–23
Cooper RA, Molan PC, Harding KG (1999) Antibacterial activity of honey against strains of Staphylococcus aureus from infected wounds. J R Soc Med 92:283–285
French VM, Cooper RM, Molan PC (2005) The antibacterial activity of honey against coagulase-negative staphylococci. J Antimicrob Chemother 56:228–231
Dröge W (2000) Free radicals in the physiological control of cell function. Physiol Rev 82:47–95
Pérez-Jiménez J, Serrano J, Tabernero M, Arranz S, Díaz-Rubio ME, García-Diz L, Goñi I, Saura-Calixto F (2009) Bioavailability of phenolic antioxidants associated with dietary fiber: Plasma antioxidant capacity after acute and long-term intake in human. Plant Foods Hum Nutr 64:102–107
Gheldof N, Engeseth NJ (2002) Antioxidant capacity of honeys from various floral sources based on the determination of oxygen radical absorbance capacity and inhibition of in vitro lipoprotein oxidation in human serum samples. J Agric Food Chem 50:3050–3055
Gheldof N, Wang XH, Engeseth NJ (2003) Buckwheat honey increases serum antioxidant capacity in humans. J Agric Food Chem 51:1500–1505
Yao LH, Jiang YM, Shi J, Tomas-Barberan FA, Datta N, Singanusong R, Chen SS (2004) Flavonoids in food and their health benefits. Plant Foods Hum Nutr 59:113–122
Busserolles J, Gueux E, Rock E, Mazur A, Rayssiguier Y (2002) Substituting honey for refined carbohydrates protects rats from hypertriglyceridemic and prooxidative effects of fructose. J Nutr 132:3379–3382
Shoham S, Youdim MB (2000) Iron involvement in neural damage and microgliosis in models of neurodegenerative diseases. Cell Mol Biol 46:743–760
Stohs SJ, Bagchi D (1995) Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med 18:321–336
Domingo JL (1994) Metal-induced developmental toxicity in mammals: A review. J Toxicol Environ Health 42:123–290
Alvarez-Suarez JM, González-Paramás AM, Santos-Buelga C, Battino M (2010) Antioxidant characterization of native monofloral Cuban honeys. J Agric Food Chem 58:9817–9824
Alvarez-Suarez JM, Tulipani S, Diaz D, Estevez Y, Romandini S, Giampieri F, Damiani E, Astolfi P, Bompadre S, Battino M (2010) Antioxidant and antimicrobial capacity of several monofloral Cuban honeys and their correlation with color, polyphenol content and other chemical compounds. Food Chem Toxicol 48:2490–2499
Louveaux J, Maurizio A, Vorwohl G (1970) Methods of melissopalynology. Bee World 51:125–138
Copper RA, Molan PC, Harding KG (2002) The sensitivity to honey of Gram-positive cocci of clinical significance isolated from wounds. J Appl Microbiol 93:857–863
Velazquez E, Tournier HA, Mordujovich de Buschiazzo P, Saavedra G, Schinella GR (2003) Antioxidant activity of Paraguayan plant extracts. Fitoterapia 74:91–97
Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG (2005) Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem 91:571–577
Janzen EG (1977) Spin Trapping. Acc Chem Res 4:31–40
Buettner GR (1987) Spin Trapping: ESR parameters of spin adduct. Free Rad Biol Med 3:259–303
Henriques A, Jacksin S, Coope R, Burton N (2006) Free radical production and quenching in honeys with wound healing potential. J Antimicrob Chemother 58:773–777
Oyanagui Y (1984) Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Anal Biochem 142:290–296
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:331–358
Jiang ZY, Hunt JV, Wolff SP (1992) Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem 202:384–389
Alvarez-Suarez JM, Tulipani S, Romandini S, Vidal A, Battino M (2009) Methodological aspects about determination of phenolic compounds and in vitro evaluation of antioxidant capacity in the honey: A review. Curr Anal Chem 5:292–303
Inoue K, Murayama S, Seshimo F, Takeba K, Yoshimura Y, Nakazawa H (2005) Identification of phenolic compound in manuka honey as specific superoxide anion radical scavenger using electron spin resonance (ESR) and liquid chromatography with coulometric array detection. J Sci Food Agric 85:872–878
Kücük M, Kolaylı S, Karaoğlu S, Ulusoy E, Baltacı C, Candan F (2007) Biological activities and chemical composition of three honeys of different types from Anatolia. Food Chem 100:526–534
Krishna-Kishorea R, Sukari-Halima A, Nurul-Syazanaa MSK, Sirajudeen NS (2011) Tualang honey has higher phenolic content and greater radical scavenging activity compared with other honey sources. Nutr Res 31:322–325
Pérez E, Rodríguez-Malaver AJ, Vit P (2006) Antioxidant capacity of Venezuelan honey in wistar rat homogenates. J Med Food 9:510–516
Fernández-Torres R, Pérez-Bernal JL, Bello-López MA, Callejón-Mochón M, Jiménez-Sánchez JC, Guiraúm-Pérez A (2005) Mineral content and botanical origin of Spanish honeys. Talanta 65:686–691
Tuzen M, Silici S, Mendil D, Soylak M (2005) Trace element levels in honeys from different regions of Turkey. Food Chem 103:325–330
Stohs SJ, Bagchi D (1995) Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med 18:321–336
Acknowledgements
The authors wish to thank the Characterization Project of Cuban Specific Honey and the Cuban National Center of Apiculture Research for their collaboration.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alvarez-Suarez, J.M., Giampieri, F., Damiani, E. et al. Radical-scavenging Activity, Protective Effect Against Lipid Peroxidation and Mineral Contents of Monofloral Cuban Honeys. Plant Foods Hum Nutr 67, 31–38 (2012). https://doi.org/10.1007/s11130-011-0268-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-011-0268-7