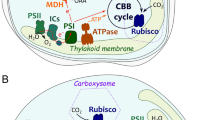
Abstract
In the genome of the marine diatom—Thalassiosira pseudonana, there are several putative genes encoding enzymes potentially constitute a classical C4 type biochemical CO2-concentrating mechanism. Two genes encode a carboxylation enzyme phosphoenolpyruvate carboxylase (PEPC)1 and PEPC2; and another two encode decarboxylation enzymes, NAD+-dependent malic enzyme (NAD-ME) and phosphoenolpyruvate carboxykinase (PEPCK). These genes were tagged by the enhanced-green fluorescence protein, egfp, ligated in the transformation vector, and transformed into the cells of T. pseudonana for localization of GFP fusion products. The PEPC1:GFP fusion was localized at the matrix of the periplastidal compartment, while the PEPC2:GFP fusion was localized at the mitochondria. The NAD-ME:GFP fusion was localized in the cytosol and the PEPCK:GFP fusion at the mitochondria. The transcripts level of NAD-ME was extremely low, and PEPCK transcript was significantly induced under the dark, suggesting that PEPCK is involved in the dark metabolism such as respiration and amino acid metabolism in the mitochondria. Treatments of low-CO2grown T. pseudonana cells with inhibitors for PEPCK and PEPC efficiently dissipated the maximum rate of photosynthesis while these treatments did not affect high-affinity photosynthesis. These data strongly suggest that classical C4 enzymes play little role in the CCM in T. pseudonana.





Similar content being viewed by others
Abbreviations
- CCM:
-
CO2-concentrating mechanism
- DIC:
-
Dissolved inorganic carbon
- PEPC:
-
Phosphoenolpyruvate carboxylase
- PEPCK:
-
Phosphoenolpyruvate carboxykinase
- NAD-ME:
-
NAD+-dependent malic enzyme
- PPDK:
-
Pyruvate phosphate dikinase
- PYC:
-
Pyruvate carboxylase
- CA:
-
Carbonic anhydrase
- 3-MPA:
-
3-Mercaptopicolinic acid
- DCDP:
-
3,3-Dichloro-2-(dihydroxyphosphinoylmethyl)propenoate
References
Allen AE, Dupont CL, Obornik M, Horak A, Nunes-Nesi A, McCrow JP, Zheng H, Johnson DA, Hu H, Fernie AR, Bowler C (2011) Evolution and metabolic significance of the urea cycle in photosynthetic diatoms. Nature 473(7346):203–207
Armbrust EV (2009) The life of diatoms in the world’s oceans. Nature 459(7244):185–192
Armbrust EV et al (2004) The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science 306(5693):79–86
Aubry S, Brown NJ, Hibberd JM (2011) The role of proteins in C3 plants prior to their recruitment into the C4 pathway. J Exp Bot 62(9):3049–3059
Badger MR, Hanson D, Price DG (2002) Evolution and diversity of CO2 concentrating mechanisms in cyanobacteria. Funct Plant Biol 29:161–173
Birmingham BC, Colman B (1979) Measurement of carbon dioxide compensation points of freshwater algae. Plant Physiol 64(5):892–895
Bowler C et al (2008) The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 456(7219):239–244
Burkhardt S, Amoroso G, Riebesell U, Sültemeyer D (2001) CO2 and HCO3 − uptake in marine diatoms acclimated to different CO2 concentrations. Limnol Oceanogr 46(6):1378–1391
Emanuelsson O, Nielsen H, Brunak S, von Heijne G (2000) Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol 300(4):1005–1016
Furbank RT (2011) Evolution of the C4 photosynthetic mechanism: are there really three C4 acid decarboxylation types? J Exp Bot 62(9):3103–3108
Gerrard Wheeler MC, Tronconi MA, Drincovich MF, Andreo CS, Flugge UI, Maurino VG (2005) A comprehensive analysis of the NADP-malic enzyme gene family of Arabidopsis. Plant Physiol 139(1):39–51
Gerrard Wheeler MCG, Arias CL, Tronconi MA, Maurino VG, Andreo CS, Drincovich MF (2008) Arabidopsis thaliana NADP-malic enzyme isoforms: high degree of identity but clearly distinct properties. Plant Mol Biol 67(3):231–242
Gould SB, Sommer MS, Hadfi K, Zauner S, Kroth PG, Maier UG (2006a) Protein targeting into the complex plastid of cryptophytes. J Mol Evol 62(6):674–681
Gould SB, Sommer MS, Kroth PG, Gile GH, Keeling PJ, Maier UG (2006b) Nucleus-to-nucleus gene transfer and protein retargeting into a remnant cytoplasm of cryptophytes and diatoms. Mol Biol Evol 23(12):2413–2422
Granum E, Roberts K, Raven JA, Leegood RC (2009) Primary carbon and nitrogen metabolic gene expression in the diatom Thalassiosira pseudonana (Bacillariophyceae): diel periodicity and effects of inorganic carbon and nitrogen. J Phycol 45(5):1083–1092
Gruber A, Vugrinec S, Hempel F, Gould SB, Maier UG, Kroth PG (2007) Protein targeting into complex diatom plastids: functional characterisation of a specific targeting motif. Plant Mol Biol 64(5):519–530
Guillard RR, Ryther JH (1962) Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can J Microbiol 8:229–239
Harrison PJ, Waters RE, Taylor FJR (1980) A broad-spectrum artificial seawater medium for coastal and open ocean phytoplankton. J Phycol 16(1):28–35
Hatch MD, Osmond CB (1976) Compartmentation and transport in C4 photosynthesis. In: Stocking SR, Hever U (eds) Encyclopedia of plant physiology, vol 3. Springer, Berlin, pp 144–184
Hempel F, Bullmann L, Lau J, Zauner S, Maier UG (2009) ERAD-derived preprotein transport across the second outermost plastid membrane of diatoms. Mol Biol Evol 26(8):1781–1790
Holdworth ES, Colbeck J (1976) The pattern of carbon fixation in the marine unicellular alga Phaeodactylum tricornutum. Mar Biol 38(2):189–199
Hopkinson BM, Dupont CL, Allen AE, Morel FMM (2011) Efficiency of the CO2-concentrating mechanism of diatoms. Proc Natl Acad Sci USA 108(10):3830–3837
Hopkinson BM, Meile C, Shen C (2013) Quantification of extracellular carbonic anhydrase in two marine diatoms and investigation of its role. Plant Physiol 162(2):1142–1152
Inui M, Dumay V, Zahn K, Yamagata H, Yukawa H (1997) Structural and functional analysis of the phosphoenolpyruvate carboxylase gene from the purple non sulfur bacterium Rhodopseudomonas palustris No.7. J Bacteriol 179(15):4942–4945
Izui K, Matsumura H, Furumoto T, Kai Y (2004) Phosphoenolpyruvate carboxylase: a new era of structural biology. Annu Rev Plant Biol 55:69–84
Jenkins CLD, Harris RLN, McFadden HG (1987) 3,3-Dichloro-2-dihydroxyphosphonoylmethyl-2-propenoate, a new specific inhibitor of phosphoenolpyruvate carboxylase. Biochem Int 14:219–226
Jitrapakdee S, Wallace JC (1999) Structure, function and regulation of pyruvate carboxylase. Biochem J 340(1):1–16
Kai Y, Matsumura H, Inoue T, Terada K, Nagara Y, Yoshinaga T, Kihara A, Tsumura K, Izui K (1999) Three-dimensional structure of phosphoenolpyruvate carboxylase: a proposed mechanism for allosteric inhibition. Proc Natl Acad Sci USA 96(3):823–828
Kilian O, Kroth PG (2005) Identification and characterization of a new conserved motif within the presequence of proteins targeted into complex diatom plastids. Plant J 41(2):175–183
Kooistra WHCF, Gersonde R, Medlin L, Mann DG (2007) The origin and evolution of the diatoms: their adaptation to a planktonic existence. In: Falkowski PG, Knoll AH (eds) Evolution of primary producers in the sea. Elsevier Academic Press, Boston, pp 207–249
Krogh A, Larsson B, von Heijne G, Sonnhammer ELL (2001) Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305(3):567–580
Kroth PG (2002) Protein transport into secondary plastids and the evolution of primary and secondary plastids. Int Rev Cytol 221:191–255
Kroth PG, Chiovitti A, Gruber A, Martin-Jezequel V, Mock T, Parker MS, Stanley MS, Kaplan A, Caron L, Weber T, Maheswari U, Armbrust EV, Bowler C (2008) A model for carbohydrate metabolism in the diatom Phaeodactylum tricornutum deduced from comparative whole genome analysis. PLoS ONE 3(1):e1426
Matsuda Y, Nakajima K, Tachibana M (2011) Recent progresses on the genetic basis of the regulation of CO2 acquisition systems in response to CO2 concentration. Photosynth Res 109:191–203
Matte A, Goldie H, Sweet RM, Delbaere LT (1996) Crystal structure of Escherichia coli phosphoenolpyruvate carboxykinase: a new structural family with the P-loop nucleoside triphosphate hydrolase fold. J Mol Biol 256(1):126–143
Maurino VG, Gerrarad Wheeler MCG, Andreo CS, Drincovich MF (2009) Redundancy is sometimes seen only by the uncritical: does Arabidopsis need six malic enzyme isoforms? Plant Sci 176(6):715–721
McGinn PJ, Morel FM (2008) Expression and inhibition of the carboxylating and decarboxylating enzymes in the photosynthetic C4 pathway of marine diatoms. Plant Physiol 146(1):300–309
Moroney JV, Ma Y, Frey WD, Fusilier KA, Pham TT, Simms TA, DiMario RJ, Yang J, Mukherjee B (2011) The carbonic anhydrase isoforms of Chlamydomonas reinhardtii: intracellular location, expression, and physiological roles. Photosynth Res 109:133–149
Moustafa A, Beszteri B, Maier UG, Bowler C, Valentin K, Bhattacharya D (2009) Genomic footprints of a cryptic plastid endosymbiosis in diatoms. Science 324(5935):1724–1726
Nakajima K, Tanaka A, Matsuda Y (2013) SLC4 family transporters in a marine diatom directly pump bicarbonate from seawater. Proc Natl Acad Sci USA 110(5):1767–1772
O’Leary B, Park J, Plaxton WC (2011) The remarkable diversity of plant PEPC (phosphoenolpyruvate carboxylase): recent insights into the physiological functions and post-translational controls of non-photosynthetic PEPCs. Biochem J 436(1):15–34
Petersen TN, Brunak S, von Heijne G, Nielsen H (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8(10):785–786
Poulsen N, Chesley PM, Kröger N (2006) Molecular genetic manipulation of the diatom Thalassiosira pseudonana (bacillariophyceae). J Phycol 42:1059–1065
Raven JA (1997) Putting the C in phycology. Eur J Phycol 32:319–333
Raven JA (2012) Algal biogeography: metagenomics show distribution of a picoplanktonic pelagophyte. Curr Biol 22:R682–R683
Ray TB, Black CC (1976) Inhibition of oxaloacetate decarboxylation during C4 photosynthesis by 3-mercaptopicolinic acid. J Biol Chem 251(18):5824–5826
Reinfelder JR, Kraepiel AM, Morel FM (2000) Unicellular C4 photosynthesis in a marine diatom. Nature 407(6807):996–999
Reinfelder JR, Milligan AJ, Morel FM (2004) The role of the C4 pathway in carbon accumulation and fixation in a marine diatom. Plant Physiol 135(4):2106–2111
Roberts K, Granum E, Leegood RC, Raven JA (2007) C3 and C4 pathways of photosynthetic carbon assimilation in marine diatoms are under genetic, not environmental, control. Plant Physiol 145(1):230–235
Samukawa M, Shen C, Hopkinson BM, Matsuda Y (2014) Localization of putative carbonic anhydrases in the marine diatom, Thalassiosira pseudonana. Photosynth Res (in press)
Sonnhammer ELL, von Heijne G, Krogh A (1998) A hidden Markov model for predicting transmembrane helices in protein sequences. In: Glasgow J, Littlejohn T, Major F, Lathrop R, Sankoff D, Sensen C (eds) Proceedings of the sixth international conference on intelligent systems for molecular biology. AAAI Press, Menlo Park, CA, pp 175–182
Sorhannus U (2007) A nuclear-encoded small-subunit ribosomal RNA timescale for diatom evolution. Mar Micropaleontol 65(1–2):1–12
Tachibana M, Allen AE, Kikutani S, Endo Y, Bowler C, Matsuda Y (2011) Localization of putative carbonic anhydrases in two marine diatoms, Phaeodactylum tricornutum and Thalassiosira pseudonana. Photosynth Res 109:205–221
Tao X, Yang Z, Tong L (2003) Crystal structures of substrate complexes of malic enzyme and insights into the catalytic mechanism. Structure 11(9):1141–1150
Tchernov D, Hassidim M, Luz B, Sukenik A, Reinhold L, Kaplan A (1997) Sustained net CO2 evolution during photosynthesis by marine microorganisms. Curr Biol 7(10):723–728
Trimborn S, Wolf-Gladrow D, Richter KU, Rost B (2009) The effect of pCO2 on carbon acquisition and intracellular assimilation in four marine diatoms. J Exp Mar Biol Ecol 376(1):26–36
Tsuji Y, Suzuki I, Shiraiwa Y (2009) Photosynthetic carbon assimilation in the coccolithophorid Emiliania huxleyi (Haptophyta): evidence for the predominant operation of the C3 cycle and the contribution of β-carboxylases to the active anaplerotic reaction. Plant Cell Physiol 50(2):318–329
Wedding RT, O’Brien CE, Kline K (1994) Oligomerization and the affinity of maize phosphoenolpyruvate carboxylase for its substrate. Plant Physiol 104(2):613–616
Wittpoth C, Kroth PG, Weyrauch K, Kowallik KV, Strotmann H (1998) Functional characterization of isolated plastids from two marine diatoms. Planta 206(1):79–85
Yang JQ, Kalhan SC, Hanson RW (2009) What is the metabolic role of phosphoenolpyruvate carboxykinase? J Biol Chem 284(40):27025–27029
Young JN, Rickaby REM, Kapralov MV, Filatov DA (2012) Adaptive signals in algal Rubisco reveal a history of ancient atmospheric carbon dioxide. Philos Trans R Soc B 367:483–492
Acknowledgments
We thank Dr. Katsura Izui for providing DCDP. We thank Ms. Nobuko Higashiuchi for her technical assistance and Ms. Miyabi Inoue for her skillful secretarial assistance. This work was supported by Grant-in-Aid for Scientific Research B (Grant no. 24310015 to Y. M.), by Grant-in-Aid for Challenging Exploratory Research (Grant no. 24651119 to Y. M.) from Japan Society for the Promotion of Science (JSPS), by MEXT-Supported Program for the Strategic Research Foundation for the Advancement of Environmental Protection Technology and for Development of Intelligent Self-Organized Biomaterials.
Author information
Authors and Affiliations
Corresponding author
Additional information
Rie Tanaka and Sae Kikutani have contributed equally to this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tanaka, R., Kikutani, S., Mahardika, A. et al. Localization of enzymes relating to C4 organic acid metabolisms in the marine diatom, Thalassiosira pseudonana . Photosynth Res 121, 251–263 (2014). https://doi.org/10.1007/s11120-014-9968-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-014-9968-9