Abstract
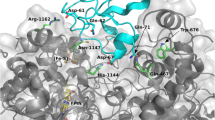
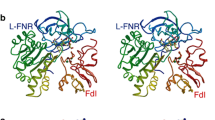
It had been proposed that a loop, typically containing 26 or 27 amino acids, which is only present in monomeric, ferredoxin-dependent, “plant-type” glutamate synthases and is absent from the catalytic α-subunits of both NADPH-dependent, heterodimeric glutamate synthases found in non-photosynthetic bacteria and NADH-dependent heterodimeric cyanobacterial glutamate synthases, plays a key role in productive binding of ferredoxin to the plant-type enzymes. Site-directed mutagenesis has been used to delete the entire 27 amino acid-long loop in the ferredoxin-dependent glutamate synthase from the cyanobacterium Synechocystis sp. PCC 6803. The specific activity of the resulting loopless variant of this glutamate synthase, when reduced ferredoxin serves as the electron donor, is actually higher than that of the wild-type enzyme, suggesting that this loop is not absolutely essential for efficient electron transfer from reduced ferredoxin to the enzyme. These results are consistent with the results of an in-silico study that suggests that the loop is unlikely to interact directly with ferredoxin in the energetically most favorable model of a 1:1 complex of ferredoxin with the wild-type enzyme.



Similar content being viewed by others
Abbreviations
- CD:
-
Circular dichroism
- Fd:
-
Ferredoxin
- FNR:
-
Ferredoxin:NADP+ oxidoreductase
- GOGAT:
-
Glutamine:oxoglutarate amidotransferase
- ICP:
-
Inductively coupled plasma
- MALDI-TOF:
-
Matrix-assisted laser desorption ionization, time-of-flight
- MS:
-
Mass spectrometry
- MV:
-
Methyl viologen
- PMSF:
-
Phenylmethanesulfonyl fluoride
- SDS-PAGE:
-
Polyacrylamide gel electrophoresis in the presence of sodium dodecylsulfate
References
Akashi T, Matsumura T, Ideguchi T, Iwakiri K, Kawakatsu T, Taniguchi I, Hase T (1999) Comparison of the electrostatic binding sites on the surface of ferredoxin for two ferredoxin-dependent enzymes, ferredoxin-NADP reductase and sulfite reductase. J Biol Chem 274:29399–293405
Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA (2001) Electrostatics of nanosystems: application to microtubules and the ribosome. Proc Natl Acad Sci USA 98:10037–10041
Binda C, Bossi RT, Wakatsuki S, Arzt S, Coda A, Curti B, Vanoni MA, Mattevi A (2000) Cross-talk and ammonia channeling between active centers in the unexpected domain arrangement of glutamate synthase. Structure 8:1299–1308
Bottin H, Lagoutte B (1992) Ferredoxin and flavodoxin from the cyanobacterium Synechocystis sp. PCC 6803. Biochim Biophys Acta 1011:48–56
Bradford MM (1976) A rapid and sensitive for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cassan N, Lagoutte B, Sétif P (2005) Ferredoxin-NADP+ reductase. Kinetics of electron transfer, transient intermediates, and catalytic activities studied by flash- absorption spectroscopy with isolated photosystem I and ferredoxin. J Biol Chem 280:25960–25972
Faeder EJ, Siegel LM (1973) A rapid micromethod for determination of FMN and FAD in mixtures. Anal Biochem 53:332–336
García-Sánchez MI, Gotor C, Jacquot J-P, Stein M, Suzuki A, Vega JM (1997) Critical residues of Chlamydomonas reinhardtii ferredoxin for interaction with nitrite reductase and glutamate synthase revealed by site-directed mutagenesis. Eur J Biochem 250:364–368
García-Sánchez MI, Díaz-Quintana A, Gotor C, Jacquot J-P, De la Rosa MA, Vega JM (2000) Homology predicted structure and functional interaction of ferredoxin from the eukaryotic alga Chlamydomonas reinhardtii with nitrite reductase and glutamate synthase. J Biol Inorg Chem 5:713–719
Hase T, Schürmann P, Knaff DB (2006) The interaction of ferredoxin with ferredoxin- dependent enzymes. In: Golbeck J (ed) Photosystem 1. Springer, Dordrecht, pp 477–498
Hirasawa M, Knaff DB (1993) The role of lysine and arginine residues at the ferredoxin- binding site of spinach glutamate synthase. Biochim Biophys Acta 1144:85–91
Hirasawa M, Boyer JM, Gray KA, Davis DJ, Knaff DB (1986) The interaction of ferredoxin with ferredoxin-dependent enzymes. Biochim Biophys Acta 851:23–28
Hirasawa M, Chang K-T, Morrow KJ Jr, Knaff DB (1989) Circular dichroism, binding and immunological studies of the interaction between spinach ferredoxin and glutamate synthase. Biochim Biophys Acta 977:150–156
Hirasawa M, Chang K-T, Knaff DB (1991) The interaction of ferredoxin and glutamate synthase: cross-linking and immunological studies. Arch Biochem Biophys 286:171–177
Kim SG, Chung J-S, Sutton RB, Lee J-S, López-Maury L, Lee SY, Florencio FJ, Lin T, Zabet-Moghaddam M, Wood MJ, Nayak K, Madem V, Tripathy JN, Kim S-K, Knaff DB (2012) Redox, mutagenic and structural studies of the glutaredoxin/arsenate reductase couple from the cyanobacterium Synechocystis sp. PCC 6803. Biochim Biophys Acta 1824:392–403
Lyskov S, Gray JJ (2008) The RosettaDock server for local protein-protein docking. Nucleic Acids Res 36:W233–W238
Muro-Pastor MI, Reyes JC, Florencio FJ (2005) Ammonium assimilation in cyanobacteria. Photosynth Res 83:135–150
Navarro F, Chávez S, Candau P, Florencio FJ (1995) Evidence of two ferredoxin-glutamate synthases in the cyanobacterium Synechocystis sp. PCC 6803. Isolation and insertional inactivation of gltB and gltS genes. Plant Mol Biol 27:753–767
Navarro F, Martin-Figueroa E, Candau P, Florencio FJ (2000) Ferredoxin-dependent iron-sulfur flavoprotein (GlsF) from the cyanobacterium Synechocystis sp. PCC 6803: expression and assembly in Escherichia coli. Arch Biochem Biophys 379:267–276
Ravasio S, Dossema L, Martin-Figguero E, Florencio FJ, Mattevi A, Morandi P, Curti B, Vanoni MA (2002) Properties of the recombinant ferredoxin-dependent glutamate synthase of Synechocystis PCC 6803. Comparison with the Azospitillum brasilense NADPH-dependent enzyme and its isolated R subunit. Biochemistry 41:8120–8133
Schmitz S, Navarro F, Kutzki CK, Florencio FJ, Böhme H (1996) Glutamate 94 of [2Fe–2S] ferredoxin is important for efficient electron transfer in the 1:1 complex formed with ferredoxin-glutamate synthase (GltS) from Synechocystis sp. PCC 6803. Biochim Biophys Acta 1227:135–140
Schrodinger L (2013) The PyMOL Molecular Graphics System, Version 1.6. Schrödinger LLL, New York
Shinmura K, Muraki N, Yoshida A, Hase T, Kurisu G (2012) Crystallization and preliminary X-ray studies of an electron transfer complex of ferredoxin and ferredoxin-dependent glutamate synthase from the cyanobacterium Leptolyngbya boryana. Acta Cryst F68:324–327
Siegel LM, Murphy MJ, Kamin H (1973) Reduced nicotinamide adenine dinucleotidephosphae-sulfite reductase of enterobacteria. I. The Escherichia coli hemoflavoprotein: molecular parameters and prosthetic groups. J Biol Chem 248:251–264
Srivastava AP, Hirasawa M, Bhalla M, Chung J-S, Allen JP, Johnson MK, Tripathy JN, Rubio LM, Vaccaro B, Subramanian S, Flores E, Zabet-Moghaddam M, Stitle K, Knaff DB (2013) The roles of four conserved basic amino acids in a ferredoxin-dependent cyanobacterial nitrate reductase. Biochemistry 52:4343–4353
Suzuki A, Knaff DB (2005) Nitrogen metabolism and roles of glutamate synthase in higher plants. Photosynth Res 83:191–217
Tovchigrechko A, Vakser IA (2006) GRAMM-X public web server for protein–protein docking. Nucleic Acids Res 34:W310–W314
van den Heuvel RHH, Ferrari D, Bossi RT, Ravasio S, Curti B, Vanoni MA, Florencio FJ, Mattevi A (2002) Structural studies on the synchronization of catalytic centers in glutamate synthase. J Biol Chem 277:24579–24583
van den Heuvel RHH, Svergun DI, Peroukhov MV, Coda A, Curti B, Ravasio S, Vanoni MA, Mattevi A (2003) The active conformation of glutamate synthase and its binding to ferredoxin. J Mol Biol 330:113–128
Vanoni MA, Curti B (1999) Glutamate synthase: a complex iron–sulfur flavoprotein. Cell Mol Life Sci 55:617–638
Vanoni MA, Curti B (2008) Structure–function studies of glutamate synthases: a class of self-regulated iron-sulfur flavoenzymes essential for nitrogen assimilation. Life 60:287–300
Vanoni MA, Verzotti E, Zanetti G, Curti B (1996) Properties of the recombinant β-subunit of glutamate synthase. Eur J Biochem 236:937–946
Vanoni MA, Fischer F, Ravasio S, Verzotti E, Edmondson DE, Hagen WR, Zanetti G, Curti B (1998) The recombinant α-subunit of glutamate synthase. Spectroscopic and catalytic properties. Biochemistry 37:1828–1838
Vigara AJ, Garcia-Sàncjez ML, Gotor C, Vega JM (1996) Interaction between glutamate synthase and ferredoxin from Monoraphidium baunii. Chemical modification and cross-linking studies. Plant Physiol Biochem 34:707–711
Xu X, Kim S-K, Schürmann P, Hirasawa M, Tripathy JN, Smith J, Knaff DB, Ubbink M (2006) Ferredoxin/ferredoxin–thioredoxin reductase complex: complete NMR. FEBS Lett 580:6714–6720
Acknowledgments
The mutagenesis, protein expression and purification, kinetic measurements, substrate-binding determinations, and data analysis carried out at Texas Tech University were funded by the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the U.S. Department of Energy, through Grant DE-FG03-99ER20346 (to D. B. K.). Sequence alignment comparisons and activity measurements on cell-free extracts of Synechocystis sp. PCC 6803 were funded by a grant (BFU2010-15708 to F. J. F.) from the Ministerio de Economia y Competitividad of Spain and European Regional Funds (FEDER). The authors would like to thank Prof. James P. Allen (Department of Chemistry and Biochemistry, Arizona State University) for carrying out the iron content determinations. The authors would also like to thank Dr. Bernard Lagoutte (CEA Saclay) for the gift of the clone used to express Synechocystis sp. PCC 6803 FNR. The authors would like to acknowledge the assistance of Ms. Mahima Kruthiventi in some of the initial mutagenesis experiments. Ms. Afia Dasgupta, Ms. Mahima Kruthiventi, and Ms. Nanditha Vaidyanathan were supported, in part, by graduate fellowships from the Texas Tech University Graduate School.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jatindra N. Tripathy and Masakazu Hirasawa contributed equally to this project.
Rights and permissions
About this article
Cite this article
Tripathy, J.N., Hirasawa, M., Sutton, R.B. et al. A loop unique to ferredoxin-dependent glutamate synthases is not absolutely essential for ferredoxin-dependent catalytic activity. Photosynth Res 123, 129–139 (2015). https://doi.org/10.1007/s11120-014-0044-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-014-0044-2