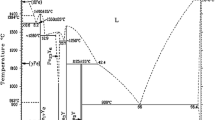
The evolution of phase composition and mechanical properties and the formation of oxide layers on Fe40–xNiCoCrAlx (x = 5 and 10 at.%) alloys in long-term oxidation at 900 and 1000°C were studied. In the initial cast state, depending on the aluminum content and valence electron concentration, the alloys contain only an fcc solid solution (VEC = 8 e/a) or a mixture of fcc and bcc phases (VEC = = 7.75 e/a). Thin continuous oxide scales containing Cr2O3 and NiCr2O4 spinel formed on the surface of both alloys oxidized at 900°C for 50 h. A further increase in the annealing time to 100 h leads to the formation of aluminum oxide Al2O3 in the scale on the Fe30Ni25Co15Cr20Al10 alloy, having high protective properties. An increase in the oxidation temperature to 1000°C results in partial failure of the protective layer on the alloy with 10 at.% Al. Long-term holding at 900°C (100 h) + 1000°C (50 h) does not change the phase composition of the Fe35Ni25Co15Cr20Al5 alloy matrix, being indicative of its high thermal stability. In the two-phase Fe30Ni25Co15Cr20Al10 alloy, the quantitative ratio of solid solutions sharply changes: the amount of the bcc phase increases from 4 to 54 wt.% and its B2-type ordering is observed. The mechanical characteristics of the starting alloys and those after long-term high-temperature annealing were determined by automated indentation. The hardness (HIT) and elastic modulus (E) of the cast Fe35Ni25Co15Cr20Al5 alloy are equal to 2 and 147 GPa, respectively, and decrease to 1.8 and 106 GPa after a series of long-term annealing operations. The Fe30Ni25Co15Cr20Al10 alloy shows the opposite dependence: HIT increases from 2.5 in the initial state to 3.1 GPa after annealing and E decreases from 152 to 134 GPa. This indicates that the Fe30Ni25Co15Cr20Al10 alloy is promising as a high-temperature oxidation-resistant and creep-resistant material.






Similar content being viewed by others
References
J.W. Yeh, S.K. Chen, S.J. Lin, J.Y. Gan, T.S. Chin, T.T. Shun, C.H. Tsau, and S.Y. Chang, “Nanostructured high-entropy alloys with multiple principal elements: novel alloy design concepts and outcomes,” Adv. Eng. Mater., 6, 299–303 (2004).
G.U. Sheng and C.T. Liu, “Phase stability in high-entropy alloys: formation of solid-solution phase or amorphous phase,” Prog. Nat. Sci. Mater. Int., No. 6, 433–446 (2011).
M.C. Gao and D.E. Alman, “Searching for next single-phase high-entropy alloy compositions,” Entropy, No. 10, 4504–4519 (2013).
S.A. Firstov, V.F. Gorban, N.A. Krapivka, and E.P. Pechkovskii, “New class of materials—high-entropy alloys and coatings,” Vestn. Tomsk. Gos. Univ., 18, Issue 4, 1938–1940 (2013).
S.A. Firstov, T.G. Rogul, N.A. Krapivka, S.S. Ponomarev, V.V. Kovylyaev, N.I. Danilenko, N.D. Bega, V.I. Danilenko, and S.I. Chugunova, “Structural features and solid-solution hardening of the high-entropy CrMnFeCoNi alloy,” Powder Metall. Met. Ceram., 55, No. 3–4, 225–235 (2016).
S.A. Firstov, T.G. Rogul, N.A. Krapivka, S.S. Ponomarev, V.V. Kovylyaev, N.D. Rudyk, M.V. Karpets, and A.N. Myslyvchenko, “Effect of the crystallization rate on the structure, phase composition, and hardness of the high-entropy AlTiVCrNbMo alloy,” Deform. Razrush. Mater., No. 10, 8–15 (2013).
F.J. Wang, Y. Zhang, and G.L. Chen, “Cooling rate and size effect on the microstructure and mechanical properties of AlCoCrFeNi high-entropy alloy,” J. Eng. Mater. Technol., No. 3, 103–105 (2009).
W.R. Wang, W.L. Wang, S.C. Wang, Y.C. Tsai, C.H. Lai, and J.W. Yeh, “Effects of Al addition on the microstructure and mechanical property of AlxCoCrFeNi high-entropy alloys,” Intermetallics, 26, 44–51 (2012).
T.M. Butler and M.L. Weaver, “Influence of annealing on the microstructures and oxidation behaviors of Al8(CoCrFeNi)92, Al15(CoCrFeNi)85, and Al30(CoCrFeNi)70 high-entropy alloys,” Metals, 6, No. 9, 222–233 (2016).
Kai Wu, C.C. Li, F.P. Cheng, K.P. Chu, R.T. Huang, L.W. Tsay, and J.J. Kai, “Air-oxidation of FeCoNiCr-based quinary high-entropy alloys at 700–900°C,” Corr. Sci., 121, 116–125 (2017).
T.M. Butler and M.L. Weaver, “Oxidation behavior of arc-melted AlCrFeCoNi multi-component high-entropy alloys,” J. Alloys Compd., 674, 229–244 (2016).
G.R. Holcomb, J. Tylczak, and C. Carney, “Oxidation of CoCrFeMnNi high-entropy alloys,” J. Met. Mater. Miner., 67, No. 10, 2326–2339 (2015).
Y.-K. Kim, Y.-A. Joo, H.S. Kim, and K.-A. Lee, “High-temperature oxidation behavior of Cr–Mn–Fe–Co–Ni high-entropy alloy,” Intermetallics, 98, 45–53 (2018).
M.V. Karpets, V.F. Gorban, O.A. Rokitska, M.O. Krapivka, O.S. Makarenko, and A.V. Samelyuk, “Features of high-temperature oxidation of high-entropy AlCrFe3CoNiCu alloy,” Powder Metall. Met. Ceram., 57, No. 3–4, 221–228 (2018).
T.M. Butler and M.L. Weaver, “Investigation of the phase stabilities in AlNiCoCrFe high-entropy alloys,” J. Alloys Compd., 691, 119–129 (2017).
S. Guo, C. Ng, J. Lu, and C.T. Liu, “Effect of valence electron concentration on stability of FCC or BCC phase in high-entropy alloys,” J. Appl. Phys., No. 10, 103–105 (2011).
S.A. Firstov, V.F. Gorban, M.V. Karpets, and E.P. Pechkovskii, “Effect of electron density on phase composition of high-entropy equiatomic alloys,” Powder Metall. Met. Ceram., 54, No. 9–10, 607–613 (2016).
M.V. Karpets, O.S. Makarenko, V.F. Gorban, M.O. Krapivka, O.A. Rokitska, and S.Yu. Makarenko, “High-temperature phase transformations in multicomponent FeCoCrNiVAl alloy,” Powder Metall. Met. Ceram., 55, No. 5–6, 361–368 (2016).
C.M. Lin and H.L. Tsai, “Evolution of microstructure, hardness, and corrosion properties of high-entropy Al0.5CoCrFeNi alloy,” Intermetallics, 19, No. 3, 288–294 (2011).
Y.F. Kao, S.K. Chen, and T.J. Chen, “Electrical, magnetic, and hall properties of AlxCoCrFeNi high-entropy alloys,” J. Alloys Compd., 509, 1607–1614 (2011).
W.R. Wang, W.L. Wang, and J.W. Yeh, “Phases, microstructure and mechanical properties of AlxCoCrFeNi high-entropy alloys at elevated temperatures,” J. Alloys Compd., 589, 143–152 (2014).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkova Metallurgiya, Vol. 59, Nos. 7–8 (534), pp. 139–151, 2020.
Rights and permissions
About this article
Cite this article
Karpets, M.V., Rokytska, O.A., Yakubiv, M.I. et al. Structural State of High-Entropy Fe40–xNiCoCrAlx Alloys in High-Temperature Oxidation. Powder Metall Met Ceram 59, 467–476 (2020). https://doi.org/10.1007/s11106-020-00180-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-020-00180-3