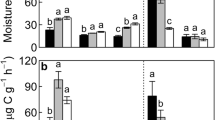
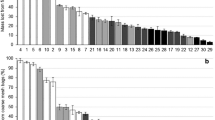
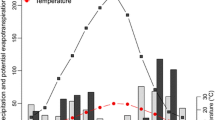
Abstract
Background and aims
Higher than expected litter decomposition rates have been observed in dry, sunny environments due to photochemical or physical degradation. However, our understanding of carbon and nutrient fluxes of standing and buried litters compared to surface litter in such areas is still scarce.
Methods
We sampled leaf litters from 51 species in a semiarid dune ecosystem and incubated them in three positions: surface, sand-buried and simulated standing.
Results
Decomposition was much faster in buried litter and somewhat faster in simulated standing litter than in surface litter. This pattern was independent of the incubation period, phylogenetic group or growth form. Litter position and incubation period significantly impacted litter nutrient dynamics. The nitrogen (N) and phosphorus (P) losses were faster in buried and simulated standing litters than in surface litter. The N loss was slower than P loss in 6-month decomposed litter but the former was relatively faster than the latter in the second phase up to 12 months of incubation.
Conclusions
Our study shows that substantial mass and nutrient losses in simulated standing and buried litters can be a candidate explanation why drylands have higher carbon and nutrient fluxes than expected based on surface litter decomposition data alone.




Similar content being viewed by others
References
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Austin AT, Ballaré CL (2010) Dual role of lignin in plant litter decomposition in terrestrial ecosystems. Proc Natl Acad Sci U S A 107:4618–4622
Austin AT, Vivanco L (2006) Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature 442:555–558
Austin AT, Yahdjian L, Stark JM, Belnap J, Porporato A, Norton U, Ravetta DA, Schaeffer SM (2004) Water pulses and biogeochemical cycles in arid and semiarid ecosystems. Oecologia 141:221–235
Austin AT, Araujo PI, Leva PE (2009) Interaction of position, litter type, and water pulses on decomposition of grasses from the semiarid Patagonian steppe. Ecology 90:2642–2647
Barnes PW, Throop HL, Hewins DB, Abbene ML, Archer SR (2012) Soil coverage reduces photodegradation and promotes the development of soil-microbial films on dryland leaf litter. Ecosystems 15:311–321
Bradford MA, Tordoff GM, Eggers T, Jones TH, Newington JE (2002) Microbiota, fauna, and mesh size interactions in litter decomposition. Oikos 99:317–323
Brandt LA, King JY, Hobbie SE, Milchunas DG, Sinsabaugh RL (2010) The role of photodegradation in surface litter decomposition across a grassland ecosystem precipitation gradient. Ecosystems 13:765–781
Breshears DD, Rich PM, Barnes FJ, Campbell K (1997) Overstory-imposed heterogeneity in solar radiation and soil moisture in a semiarid woodland. Ecol Appl 7:1201–1215
Brovkin V, van Bodegom PM, Kleinen T, Wirth C, Cornwell WK, Cornelissen JHC, Kattge J (2012) Plant-driven variation in decomposition rates improves projections of global litter stock distribution. Biogeosciences 9:565–576
Cadisch G, Giller KE (1997) Driven by nature: plant litter quality and decomposition. CAB international, Oxon
Core Team R (2012) R: A Language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2007, ISBN 3-900051-07-0
Cornelissen JHC, Perez-Harguindeguy N, Diaz S, Grime JP, Marzano B, Cabido M, Vendramini F, Cerabolini B (1999) Leaf structure and defence control litter decomposition rate across species and life forms in regional floras on two continents. New Phytol 143:191–200
Cornelissen JHC, Lavorel S, Garnier E, Diaz S, Buchmann N, Gurvich DE, Reich PB, ter Steege H, Morgan HD, van der Heijden MGA, Pausas JG, Poorter H (2003) A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust J Bot 51:335–380
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Perez-Harguindeguy N, Quested HM, Santiago LS, Wardle DA, Wright IJ, Aerts R, Allison SD, van Bodegom P, Brovkin V, Chatain A, Callaghan TV, Diaz S, Garnier E, Gurvich DE, Kazakou E, Klein JA, Read J, Reich PB, Soudzilovskaia NA, Vaieretti MV, Westoby M (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071
Coûteaux MM, Bottner P, Berg B (1995) Litter decomposition, climate and litter quality. Trends Ecol Evol 10:63–66
Coûteaux MM, McTiernan K, Berg B, Szuberla D, Dardenne P, Bottner P (1998) Chemical composition and carbon mineralisation potential of Scots pine needles at different stages of decomposition. Soil Biol Biochem 30:583–595
Dukes JS, Field CB (2000) Diverse mechanisms for CO2 effects on grassland litter decomposition. Glob Chang Biol 6:145–154
Elkins NZ, Whitford WG (1982) The role of microarthropods and Nematodes in decomposition in a semi-arid ecosystem. Oecologia 55:303–310
Fortunel C, Garnier E, Joffre R, Kazakou E, Quested H, Grigulis K, Lavorel S, Ansquer P, Castro H, Cruz P (2009) Leaf traits capture the effects of land use changes and climate on litter decomposability of grasslands across Europe. Ecology 90:598–611
Fukushima RS, Hatfield RD (2004) Comparison of the acetyl bromide spectrophotometric method with other analytical lignin methods for determining lignin concentration in forage samples. J Agric Food Chem 52:3713–3720
Gallo M, Sinsabaugh R, Cabaniss S (2006) The role of ultraviolet radiation in litter decomposition in arid ecosystems. Appl Soil Ecol 34:82–91
Güsewell S (2004) N: P ratios in terrestrial plants: variation and functional significance. New Phytol 164:243–266
Güsewell S, Verhoeven JTA (2006) Litter N: P ratios indicate whether N or P limits the decomposability of graminoid leaf litter. Plant Soil 287:131–143
Hewins DB, Archer SR, Okin GS, McCulley RL, Throop HL (2013) Soil-litter mixing accelerates decomposition in a Chihuahuan desert grassland. Ecosystems 16:183–195
Huang YH, Li YL, Xiao Y, Wenigmann KO, Zhou GY, Zhang DQ, Wenigmann M, Tang XL, Liu JX (2011) Controls of litter quality on the carbon sink in soils through partitioning the products of decomposing litter in a forest succession series in South China. For Ecol Manag 261:1170–1177
King JY, Brandt LA, Adair EC (2012) Shedding light on plant litter decomposition: advances, implications and new directions in understanding the role of photodegradation. Biogeochemistry 111:57–81
Klotzbucher T, Kaiser K, Guggenberger G, Gatzek C, Kalbitz K (2011) A new conceptual model for the fate of lignin in decomposing plant litter. Ecology 92:1052–1062
Koerselman W, Meuleman AFM (1996) The vegetation N: P ratio: a new tool to detect the nature of nutrient limitation. J Appl Ecol 33:1441–1450
Lee H, Fitzgerald J, Hewins DB, McCulley RL, Archer SR, Rahn T, Throop HL (2014) Soil moisture and soil-litter mixing effects on surface litter decomposition: a controlled environment assessment. Soil Biol Biochem 72:123–132
Li JW, Ziegler SE, Lane CS, Billings SA (2013) Legacies of native climate regime govern responses of boreal soil microbes to litter stoichiometry and temperature. Soil Biol Biochem 66:204–213
Liu GF, Freschet GT, Pan X, Cornelissen JHC, Li Y, Dong M (2010) Coordinated variation in leaf and root traits across multiple spatial scales in Chinese semi-arid and arid ecosystems. New Phytol 188:543–553
Liu GF, Cornwell WK, Pan X, Cao KF, Ye XH, Huang ZY, Dong M, Cornelissen JHC (2014) Understanding the ecosystem implications of the angiosperm rise to dominance: leaf litter decomposability among magnoliids and other basal angiosperms. J Ecol 102:337–344
Makkonen M, Berg MP, Handa IT, Hättenschwiler S, Ruijven J, Bodegom PM, Aerts R (2012) Highly consistent effects of plant litter identity and functional traits on decomposition across a latitudinal gradient. Ecol Lett 15:1033–1041
Meentemeyer V (1978) Macroclimate and lignin control of litter decomposition rates. Ecology 59:465–472
Moore TR, Trofymow JA, Prescott CE, Titus BD, Grp CW (2011) Nature and nurture in the dynamics of C, N and P during litter decomposition in Canadian forests. Plant Soil 339:163–175
Moorhead DL, Reynolds JF (1989) Mechanisms of surface litter mass-loss in the Northern Chihuahuan desert - a reinterpretation. J Arid Environ 16:157–163
Moorhead DL, Reynolds JF (1993) Changing carbon-chemistry of buried Creosote bush litter during decomposition in the Northern Chihuahuan desert. Am Midl Nat 130:83–89
Mooshammer M, Wanek W, Schnecker J, Wild B, Leitner S, Hofhansl F, Blochl A, Hammerle I, Frank AH, Fuchslueger L, Keiblinger KM, Zechmeister-Boltenstern S, Richter A (2012) Stoichiometric controls of nitrogen and phosphorus cycling in decomposing beech leaf litter. Ecology 93:770–782
Noy-Meir I (1973) Desert ecosystems: environment and producers. Annu Rev Ecol Syst 4:25–51
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:322–331
Pakeman RJ, Eastwood A, Scobie A (2011) Leaf dry matter content as a predictor of grassland litter decomposition: a test of the ‘mass ratio hypothesis’. Plant Soil 342:49–57
Parton W, Silver WL, Burke IC, Grassens L, Harmon ME, Currie WS, King JY, Adair EC, Brandt LA, Hart SC, Fasth B (2007) Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 315:361–364
Pozo J, Colino R (1992) Decomposition processes of Spartina maritima in a salt marsh of the Basque country. Hydrobiologia 231:165–175
Romero LM, Smith TJ, Fourqurean JW (2005) Changes in mass and nutrient content of wood during decomposition in a south Florida mangrove forest. J Ecol 93:618–631
Rozema J, van de Staaij J, Bjorn LO, Caldwell M (1997) UV-B as an environmental factor in plant life: stress and regulation. Trends Ecol Evol 12:22–28
Santos PF, Phillips J, Whitford WG (1981) The role of Mites and Nematodes in early stages of buried litter decomposition in a desert. Ecology 62:664–669
Santos PF, Elkins NZ, Steinberger Y, Whitford WG (1984) A comparison of surface and buried Larrea tridentata leaf litter decomposition in North-American hot deserts. Ecology 65:278–284
Sitch S, Smith B, Prentice IC, Arneth A, Bondeau A, Cramer W, Kaplan JO, Levis S, Lucht W, Sykes MT, Thonicke K, Venevsky S (2003) Evaluation of ecosystem dynamics, plant geography and terrestrial carbon cycling in the LPJ dynamic global vegetation model. Glob Chang Biol 9:161–185
Strickland MS, Osburn E, Lauber C, Fierer N, Bradford MA (2009) Litter quality is in the eye of the beholder: initial decomposition rates as a function of inoculum characteristics. Funct Ecol 23:627–636
Swift MJ, Heal OW, Anderson JM (1979) Decomposition in terrestrial ecosystems. University of California Press, Berkeley, pp 167–219
Taylor BR, Parkinson D, Parsons WFJ (1989) Nitrogen and lignin content as predictors of litter decay rates - a microcosm test. Ecology 70:97–104
Throop HL, Archer SR (2007) Interrelationships among shrub encroachment, land management, and litter decomposition in a semidesert grassland. Ecol Appl 17:1809–1823
Throop HL, Archer SR (2008) Shrub (Prosopis velutina) encroachment in a semidesert grassland: spatial-temporal changes in soil organic carbon and nitrogen pools. Global Chang Biol 14:2420–2431
van der valk AG, Attiwill PM (1983) Above-ground and below-ground litter decomposition in an Australian salt-marsh. Aust J Ecol 8:441–447
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass-C. Soil Biol Biochem 19:703–707
Vivanco L, Austin AT (2006) Intrinsic effects of species on leaf litter and root decomposition: a comparison of temperate grasses from North and South America. Oecologia 150:97–107
Westerman RL (1990) Soil testing and plant analysis. Soil science society of america, 3rd edn. Book Ser SSSA, Madison
Whitford WG, Meentemeyer V, Seastedt TR, Cromack K, Crossley DA, Santos P, Todd RL, Waide JB (1981) Exceptions to the AET model - deserts and clear-cut forest. Ecology 62:275–277
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas ML, Niinemets U, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428:821–827
Zhang DQ, Hui DF, Luo YQ, Zhou GY (2008) Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors. J Plant Ecol 1:85–93
Zhou GY, Guan LL, Wei XH, Tang XL, Liu SG, Liu JX, Zhang DQ, Yan JH (2008) Factors influencing leaf litter decomposition: an intersite decomposition experiment across China. Plant Soil 311:61–72
Acknowledgments
We thank Dr. Ruiru Gao at Institute of Botany, Chinese Academy of Sciences (CAS) for his many suggestions for species identification. We give special thanks to Mr. Zhihua Liu for his substantial help in leaf litter collecting. We thank Dr. Fangli Luo at Beijing Forestry University for her help in measuring light interception by the litterbag. We also thank Ordos Sandland Ecological Research Station in Mu Us sandland, CAS for providing meteorological data including precipitation, temperature, frozen soil layer, wind speed, and solar radiation for the whole experimental year. In addition, we thank several college students at Changchun Normal University, China including Jianguo Chen, Xing Fu, Wan Zhang and Yinyan Xie for their help in cleaning litterbags. This study was supported by the National Natural Science Foundation of China (NSFC, 31100333, 31470712) and the Young Talents in State Key Laboratory of Vegetation and Environmental Change, Institute of Botany, CAS (80006 F2059) and partly supported by Laboratory Open Fund in Institute of Grassland Science, Northeast Normal University, China. JHCC, WKC, MD, GL and XP were partly funded by the Royal Netherlands Academy of Arts and Sciences (KNAW, Chinese Exchange Programme grant 12CDP007). FL was partly funded by NSFC (grant no. 31100300). MD was partly funded by NSFC (grant no. 31261120580) and the Innovative R & D grant of Hangzhou Normal University, China (grant no. PD12002002004001).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Fernando T. Maestre
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. A1
Mean wind velocity (a), and light intensity including Ultraviolet (UV, b) and Photosynthetically active radiation (PAR, c) from November 2011 to November 2012, and relative humidity among litter positions (d) for litter incubation site from September to the end of October 2012. The solid line denotes the fitted local smoothing spline. (DOCX 120 kb)
Fig. A2
A line plot (mean ± SE, n = 51) of fraction of leaf litter mass loss after 0, 6, 9 and 12 months. Same lowercase letter for each harvest indicates no significant difference in fractions of leaf litter mass loss among litter positions at P <0.05. (DOCX 58 kb)
Fig. A3
Bar plots (mean ± SE, n = 51) of the total carbon concentration (% C) (a), total nitrogen concentration (% N) (b), total phosphorus concentration (P) (c), C/N ratio (d), N/P ratio (e), and C/P ratio (f) of half-year-decomposed and one-year-decomposed litters among three litter positions including simulated standing, surface and sand burial conditions. Same lowercase letter denotes no significant difference in nutrient concentration at P <0.05. The horizontal gray line denotes nutrient concentration or stoichiometry of initial leaf litter. The values with asterisk (*) denotes a significant difference in carbon and nutrient concentration compared with initial leaf litter at P <0.05. (DOCX 133 kb)
Fig. A4
Average temperatures of atmosphere (a), surface (b) and 10 cm soil depth (c) and the depth of frozen soil layer (d) during the period from November 2011 to October 2012. (DOCX 376 kb)
Fig. A5
The variation in total solar radiation (Eg), photosynthetically active radiation (PAR) and the pattern of ultraviolet (UV) radiation during the period from November 2011 to October 2012. (DOCX 151 kb)
Table A1
All species list in this study and their corresponding families, growth form (one of tree, shrub, grass, herbaceous legume and forb) and phylogenetic group (one of fern, gymnosperm, monocot and eudicot). (DOCX 21 kb)
Data Accessibility
Uploaded as online supporting information (“PLSO-D-15-00316.data.xlsx”). (XLSX 57 kb)
Rights and permissions
About this article
Cite this article
Liu, G., Cornwell, W.K., Pan, X. et al. Decomposition of 51 semidesert species from wide-ranging phylogeny is faster in standing and sand-buried than in surface leaf litters: implications for carbon and nutrient dynamics. Plant Soil 396, 175–187 (2015). https://doi.org/10.1007/s11104-015-2595-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2595-1