Abstract
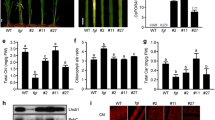
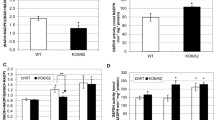
NADPH:protochlorophyllide oxidoreductase (POR) is a key enzyme for the light-induced greening of etiolated angiosperm plants. It belongs to the ‘RED’ family of reductases, epimerases and dehydrogenases. All POR proteins characterized so far contain evolutionarily conserved cysteine residues implicated in protochlorophyllide (Pchlide)-binding and catalysis. cDNAs were constructed by site-directed mutagenesis that encode PORB mutant proteins with defined Cys→Ala exchanges. These cDNAs were expressed in transgenic plants of a PORB-deficient knock-out mutant (porB) of Arabidopsis thaliana. Results show that porB plants expressing PORB mutant proteins with Ala substitutions of Cys276 or Cys303 are hypersensitive to high-light conditions during greening. Hereby, failure to assemble higher molecular weight complexes of PORB with its twin isoenzyme, PORA, as encountered with (Cys303→Ala)-PORB plants, caused more severe effects than replacing Cys276 by an Ala residue in the active site of the enzyme, as encountered in (Cys276→Ala)-PORB plants. Our results are consistent with the presence of two distinct pigment binding sites in PORB, with Cys276 establishing the active site of the enzyme and Cys303 providing a second, low affinity pigment binding site that is essential for the assembly of higher molecular mass light-harvesting PORB::PORA complexes and photoprotection of etiolated seedlings. Failure to assemble such complexes provoked photodynamic damage through the generation of singlet oxygen. Together, our data highlight the importance of PORB for Pchlide homoeostasis and greening in Arabidopsis.







Similar content being viewed by others
References
Abrahams BF, Hoskins BF, Michail DM, Robson R (1994) Assembly of porphyrin building blocks into network structures with large channels. Nature 369:727–729
Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, Gadrinab C, Heller C, Jeske A, Koesema E, Meyers CC, Parker H, Prednis L, Ansari Y, Choy N, Deen H, Geralt M, Hazari N, Hom E, Karnes M, Mulholland C, Ndubaku R, Schmidt I, Guzman P, Aguilar-Henonin L, Schmid M, Weigel D, Carter DE, Marchand T, Risseeuw E, Brogden D, Zeko A, Crosby WL, Berry CC, Ecker JR (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301:653–657
Armstrong GA, Runge S, Frick G, Sperling U, Apel K (1995) Identification of NADPH:protochloro-phyllide oxidoreductases A and B: a branched pathway for light-dependent chlorophyll biosynthesis in Arabidopsis thaliana. Plant Physiol 108(4):1505–1517
Armstrong GA, Apel K, Rüdiger W (2000) Does a light-harvesting protochlorophyllide a/b-binding protein complex exist? Trends Plant Sci 5(1):40–44
Baker ME (1994) Protochlorophyllide reductase is homologous to human carbonyl reductase and pig 20-beta-hydroxysteroid dehydrogenase. Biochem J 300:605–607
Beale SI (1999) Enzymes of chlorophyll biosynthesis. Photosynth Res 60:43–73
Benli M, Schulz R, Apel K (1991) Effect of light on the NADPH-protochlorophyllide oxidoreductase of Arabidopsis thaliana. Plant Mol Biol 16:615–625
Birve SJ, Selstam E, Johansson LB-A (1996) Secondary structure of NADPH:protochlorophyllide oxidoreductase examined by circular dichroism and prediction methods. Biochem J 317:549–555
Boardman NK (1962) Studies on a protochlorophyll-protein complex. I. Purification and molecular- weight determination. Biochem Biophys Acta 62: 63–79
Böddi B, Lindsten A, Ryberg M, Sundqvist C (1989) On the aggregational states of protochlorophyllide and its protein complexes in wheat etioplasts. Physiol Plant 76:135–143
Böddi B, Ryberg M, Sundqvist C (1993) Analysis of the 77 K fluorescence emission and excitation spectra of isolated etioplast inner membranes. J Photoch Photobio B 21:125–133
Böddi B, Kis-Petik K, Kaposi AD, Fidy J, Sundqvist C (1998) The two spectroscopically different short wavelength protochlorophyllide forms in pea epicotyls are both monomeric. Biochim Biophys Acta Bioenergetics 1365:531–540
Brzezowski P, Richter AS, Grimm B (2015) Regulation and function of tetrapyrrole biosynthesis in plants and algae. Biochim Biophys Acta 1847(9):968–985
Buhr F, El Bakkouri M, Lebedev N, Pollmann S, Reinbothe S, Reinbothe C (2008) Photoprotective role of NADPH:protochlorophyllide oxidoreductase A. Proc Natl Acad Sci USA 105:12629–12634
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Franck F, Sperling U, Frick G, Pochert B, van Cleve B, Apel K, Armstrong GA (2000) Regulation of etioplast pigment-protein complexes, inner membrane architecture, and protochlorophyllide a chemical heterogeneity by light-dependent NADPH:protochlorophyllide oxidoreductases A and B. Plant Physiol 124(4):1678–1696
Frick G, Su Q, Apel K, Armstrong GA (2003) An Arabidopsis porB porC double mutant lacking light-dependent NADPH:protochlorophyllide oxidoreductases B and C is highly chlorophyll-deficient and developmentally arrested. Plant J 35(2):141–153
Gabruk M, Stecka A, Strzałka W, Kruk J, Strzałka K, Mysliwa-Kurdziel B (2015) Photoactive protochlorophyllide-enzyme complexes reconstituted with PORA, PORB and PORC proteins of A. thaliana: fluorescence and catalytic properties. PLoS ONE 10(2):e0116990
Garrone A, Archipowa N, Zipfel PF, Hermann G, Dietzek B (2015) Plant protochlorophyllide oxidoreductases A and B: catalytic efficiency and initial reaction steps. J Biol Chem 290(47):28530–28539
Goslings D, Meskauskiene R, Kim C, Lee KP, Nater M, Apel K (2004) Concurrent interactions of heme and FLU with Glu-tRNA reductase (HEMA1), the target of metabolic feedback inhibition of tetrapyrrole biosynthesis, in dark- and light-grown Arabidopsis plants. Plant J 40:957–967
Griffiths WT (1975) Characterization of the terminal stage of chlorophyll(ide) synthesis in etioplast membrane preparations. Biochem J 152:623–635
Hanf R, Fey S, Dietzek B, Schmitt M, Reinbothe C, Reinbothe S, Hermann G, Popp J (2011) Protein-induced excited-state dynamics of protochlorophyllide. J Phys Chem A 115(27):7873–7881
Hellens RP, Edwards EA, Leyland NR, Bean S, Mullineaux PM (2000) pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol Biol 42:819–832
Heyes DJ, Hunter CN (2002) Site-directed mutagenesis of Tyr-189 and Lys-193 in NADPH: protochlorophyllide oxidoreductase from Synechocystis. Biochem Soc Trans 30:601–604
Heyes DJ, Hunter CN (2005) Making light work of enzyme catalysis: protochlorophyllide oxidoreductase. Trends Biochem Sci 30:642–649
Heyes DJ, Scrutton NS (2009) Conformational changes in the catalytic cycle of protochlorophyllide oxidoreductase: what lessons can be learnt from dihydrofolate reductase? Biochem Soc Trans 37(Pt 2):354–357
Heyes DJ et al (2007) Laser excitation studies of the product release steps in the catalytic cycle of the light-driven enzyme, protochlorophyllide oxidoreductase. J Biol Chem 282:32015–32020
Heyes DJ et al (2008) Conformational events during ternary enzyme-substrate complex formation are rate limiting in the catalytic cycle of the light-driven enzyme protochlorophyllide oxidoreductase. BioChemistry 47:10991–10998
Hideg E, Kálai T, Hideg K, Vass I (1998) Photoinhibition of photosynthesis in vivo results in singlet oxygen production detection via nitroxide-induced fluorescence quenching in broad bean leaves. BioChemistry 37:11405–11411
Holtorf H, Reinbothe S, Reinbothe C, Bereza B, Apel K (1995) Two routes of chlorophyllide synthesis that are differentially regulated by light in barley (Hordeum vulgare L.). Proc Natl Acad Sci USA 92:3254–3258
Kálai T, Hankovszky O, Hideg E, Jeko J, Hideg K (2002) Synthesis and structure optimization of double (fluorescent and spin) sensor molecules. ARKIVOC iii:112–120
Kim C, Apel K (2004) Substrate-dependent and organ-specific chloroplast protein import in planta. Plant Cell 16:88–98
Kim C, Apel K (2013) Singlet oxygen-mediated signaling in plants: moving from flu to wild type reveals an increasing complexity. Photosynth Res 116:455–464
Kim C, Ham H, Apel K (2005) Multiplicity of different cell- and organ-specific import routes for the NADPH-protochlorophyllide oxidoreductases A and B in plastids of Arabidopsis seedlings. Plant J 42(3):329–340
Klement H, Oster U, Rudiger W (2000) The influence of glycerol and chloroplast lipids on the spectral shifts of pigments associated with NADPH: Protochlorophyllide oxidoreductase from Avena sativa L. FEBS Lett. 480:306–310
Kósa A, Marton Z, Solymosi K, Boka K, Boddi B (2006) Aggregation of the 636 nm emitting monomeric protochlorophyllide form into flash-photoactive, oligomeric 644 and 655 nm emitting forms in vitro. Biochim Biophys Acta 1757:811–820
Kotzabasis K, Senge M, Seyfried B, Senger H (1990) Aggregation of monovinyl- and divinyl- protochlorophyllide in organic solvents. Photochem Photobiol 52:95–101
Kruk J, Mysliwa-Kurdziel B (2004) Separation of monovinyl and divinyl protochlorophyllides using C30-reverse phase high-performance liquid chromatography column: analytical and preparative applications. Chromatographia 60:117–123
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lebedev N, Timko MP (1998) Protochlorophyllide photoreduction. Photosynth Res 58:5–23
Lebedev N, Timko MP (1999) Protochlorophyllide oxidoreductase B-catalyzed protochlorophyllide photoreduction in vitro: insight into the mechanism of chlorophyll formation in light-adapted plants. Proc Natl Acad Sci USA 96:9954–9959
Lebedev N, van Cleve B, Armstrong GA, Apel K (1995) Chlorophyll biosynthesis in a deetiolated (det340) mutant of Arabidopsis without NADPH-protochlorophyllide (Pchlide) oxidoreductase (POR) A and photoactive Pchlide-F655. Plant Cell 7:2081–2090
Lebedev N, Karginova O, McIvor W, Timko MP (2001) Tyr275 and Lys279 stabilize NADPH within the catalytic site of NADPH:protochlorophyllide oxidoreductase and are involved in the formation of the enzyme photoactive state. BioChemistry 40:12562–12574
Masuda T, Fujita Y (2008) Regulation and evolution of chlorophyll metabolism. Photochem Photobiol Sci 7(10):1131–1149
Masuda T, Fusada N, Oosawa N, Takamatsu K, Yamamoto YY, Ohto M, Nakamura K, Goto K, Shibata D, Shirano Y, Hayashi H, Kato T, Tabata S, Shimada H, Ohta H, Takamiya K (2003) Functional analysis of isoforms of NADPH: protochlorophyllide oxidoreductase (POR), PORB and PORC, in Arabidopsis thaliana. Plant Cell Physiol 44(10):963–974
Matringe M, Camadro M, Labbe P, Scalla R (1989) Protoporphyrinogen oxidase as molecular target for diphenyl ether herbicides. Biochem J 260:231–235
Meskauskiene R, Apel K (2002) Interaction of FLU, a negative regulator of tetrapyrrole biosynthesis, with glutamyl-tRNA reductase requires the tetratricopeptide repeat domain of FLU. FEBS Lett 532:27–30
Meskauskiene R, Nater M, Goslings D, Kessler F, op den Camp R, Apel K (2001) Flu: a negative regulator of chlorophyll biosynthsis in Arabidopsis thaliana. Proc Natl Acad Sci USA 98:12826–12831
Mochizuki N, Tanaka R, Grimm B, Masuda T, Moulin M, Smith AG, Tanaka A, Terry MJ (2010) The cell biology of tetrapyrroles: a life and death struggle. Trends Plant Sci 15(9):488–498
Mock H-P, Keetman U, Kruse E, Rank B, Grimm B (1998) Defense resonses to tetrapyrrole-induced oxidative stress in transgenic plants with reduced uroporphyrinogen decarboxylase or coproporphyrinogen oxidase activity. Plant Physiol 116:107–116
Mysliwa-Kurdziel B, Kruk J, Strzałka K (2004) Fluorescence lifetimes and spectral properties of protochlorophyllide in organic solvents and their relations to the respective parameters in vivo. Photochem Photobiol 79:62–67
Mysliwa-Kurdziel B, Kruk J, Strzałka K (2013) Protochlorophyllide in model systems—an approach to in vivo conditions. Biophys Chem 175–176:28–38
Norton JD (1966) Testing of plum pollen viability with tetrazolium salts. Am Soc Hortic Sci 89:132–134
Oosawa N et al (2000) Identification and light-induced expression of a novel gene of NADPH-protochlorophyllide oxidoreductase isoform in Arabidopsis thaliana. FEBS Lett 474:133–136
op den Camp RGL, Przybyla D, Ochsenbein C, Laloi C, Kim C, Danon A, Wagner D, Hideg E, Göbel C, Feussner I, Nater M, Apel K (2003) Rapid induction of distinct stress responses after release of singlet oxygen in Arabidopsis. Plant Cell 15:2320–2332
Ouazzani Chahdi MA, Schoefs B, Franck F (1998) Isolation and characterization of photoactive complexes of NADPH:protochlorophyllide oxidoreductase from wheat. Planta 206:673–680
Paddock TN, Mason ME, Lima DF, Armstrong GA (2010) Arabidopsis protochlorophyllide oxidoreductase A (PORA) restores bulk chlorophyll synthesis and normal development to a porB porC double mutant. Plant Mol Biol 72(4–5):445–457
Paddock T, Lima D, Mason ME, Apel K, Armstrong GA (2012) Arabidopsis light-dependent protochlorophyllide oxidoreductase A (PORA) is essential for normal plant growth and development. Plant Mol Biol 78(4–5):447–460
Pattanayak GK, Tripathy BC (2002) Catalytic function of a novel protein protochlorophyllide oxidoreductase C of Arabidopsis thaliana. Biochem Biophys Res Commun 291:921–924
Reinbothe S, Reinbothe C (1996) The regulation of enzymes involved in chlorophyll biosynthesis. Eur J Biochem 237:323–343
Reinbothe S, Runge S, Reinbothe C, van Cleve B, Apel K (1995a) A Substrate-dependent transport of the NADPH:protochlorophyllide oxidoreductase into isolated plastids. Plant Cell 7:161–172
Reinbothe S, Reinbothe C, Holtorf H, Apel K (1995b) Two NADPH:protochlorophyllide oxidoreductases in barley: evidence for the selective disappearance of PORA during the light-induced greening of etiolated seedlings. Plant Cell 7:1933–1940
Reinbothe C, Apel K, Reinbothe S (1995c) A light-induced protease from barley plastids degrades NADPH:protochlorophyllide oxidoreductase complexed with chlorophyllide. Mol Cell Biol 15:6206–6212
Reinbothe S, Reinbothe C, Neumann D, Apel K (1996a) A plastid enzyme arrested in the step of precursor translocation in vivo. Proc Natl Acad Sci USA 93:12026–12030
Reinbothe S, Reinbothe C, Lebedev N, Apel K (1996b) PORA and PORB, two light-dependent protochlorophyllide-reducing enzymes of angiosperm chlorophyll biosynthesis. Plant Cell 8:763–769
Reinbothe C, Lebedev N, Apel K, Reinbothe S (1997) Regulation of chloroplast protein import through a protochlorophyllide-responsive transit peptide. Proc Natl Acad Sci USA 94:8890–8894
Reinbothe C, Lebedev N, Reinbothe S (1999) A protochlorophyllide light-harvesting complex involved in de-etiolation of higher plants. Nature 397:80–84
Reinbothe C, Mache R, Reinbothe S (2000) A second, substrate-dependent site of protein import into chloroplasts. Proc Natl Acad Sci USA 97:9795–9800
Reinbothe C, Buhr F, Pollmann S, Reinbothe S (2003a) In vitro reconstitution of light-harvesting POR-protochlorophyllide complex with protochlorophyllides a and b. J Biol Chem 278:807–815
Reinbothe S, Pollmann S, Reinbothe C (2003b) In situ conversion of protochlorophyllide b to protochlorophyllide a in barley. J Biol Chem 278:800–806
Reinbothe C, Lepinat A, Deckers M, Beck E, Reinbothe S (2003c) The extra loop distinguishing POR from the structurally related short-chain alcohol dehydrogenases is dispensable for pigment binding but needed for the assembly of light-harvesting POR-protochlorophyllide complex. J Biol Chem 278:816–822
Reinbothe S, Quigley F, Gray J, Schemenewitz A, Reinbothe C (2004a) Identification of plastid envelope proteins required for the import of protochlorophyllide oxidoreductase (POR) A into the chloroplast of barley. Proc Natl Acad Sci USA 101:2197–2202
Reinbothe S, Quigley F, Springer A, Schemenewitz A, Reinbothe C (2004b) The outer plastid envelope protein Oep16: a novel role as precursor translocase in import of protochlorophyllide oxidoreductase A. Proc Natl Acad Sci USA 101:2203–2208
Reinbothe C, Buhr F, Desvignes C, Quigley F, Pésey H, Reinbothe S (2006) In vitro mutagenesis of PORB: two distinct protochlorophyllide binding sites participate in enzyme catalysis and assembly. Mol Genet Genom 275:540–552
Reinbothe C, El Bakkouri M, Buhr F, Muraki N, Nomata J, Kurisu G, Fujita Y, Reinbothe S (2010a) Chlorophyll biosynthesis: spotlight on protochlorophyllide reduction. Trends Plant Sci 15:614–624
Reinbothe C, Pollmann S, Reinbothe S (2010b) Singlet oxygen links photosynthesis to translation and plant growth. Trends Plant Sci 15:499–506
Runge S, Sperling U, Frick G, Apel K, Armstrong GA (1996) Distinct roles for light-dependent NADPH:protochlorophyllide oxidoreductases (POR) A and B during greening in higher plants. Plant J 9(4):513–523
Ryberg M, Sundqvist C (1982) Spectral forms of protochlorophyllide in prolamellar bodies and prothylakoids fractionated from wheat etioplasts. Physiol Plant 56:133–138
Samol I, Buhr F, Springer A, Pollmann S, Lahroussi A, Rossig C, von Wettstein D, Reinbothe C, Reinbothe S (2011a) Implication of the oep16-1 mutation in a flu-independent, singlet oxygen-regulated cell death pathway in Arabidopsis thaliana. Plant Cell Physiol 52:84–95
Samol I, Rossig C, Buhr F, Springer A, Pollmann S, Lahroussi A, von Wettstein D, Reinbothe C, Reinbothe S (2011b) The outer chloroplast envelope protein OEP16-1 for plastid import of NADPH:protochlorophyllide oxidoreductase A in Arabidopsis thaliana. Plant Cell Physiol 52:96–111
Satoh H, Nakayama K, Okada M (1998) Molecular cloning and functional expression of a water-soluble chlorophyll protein, a putative carrier of chlorophyll molecules in cauliflower. J Biol Chem 273:30568–30575
Schulz R, Steinmüller K, Klaas M, Forreiter C, Rasmussen S, Hiller C, Apel K (1989) Nucleotide sequence of a cDNA coding for the NADPH-protochlorophyllide oxidoreductase (PCR) of barley (Hordeum vulgare L.) and expression in Escherichia coli. Mol Gen Genet 217:355–361
Seliskar CJ, Ke B (1968) Protochlorophyllide aggregation in solution and associated spectral changes. Biochim Biophys Acta 153:685–691
Southern E (2006) Southern blotting. Nat Protoc 1:518–525
Sperling U, van Cleve B, Frick G, Apel K, Armstrong GA (1997) Overexpression of light-dependent PORA or PORB in plants depleted of endogenous POR by far-red light enhances seedling survival in white light and protects against photooxidative damage. Plant J 12(3):649–658
Sperling U, Franck F, van Cleve B, Frick G, Apel K, Armstrong GA (1998) Etioplast differentiation in arabidopsis: both PORA and PORB restore the prolamellar body and photoactive protochlorophyllide-F655 to the cop1 photomorphogenic mutant. Plant Cell 10(2):283–296
Su Q et al (2001) PORC of Arabidopsis thaliana: a third light- and NADPH-dependent protochlorophyllide oxidoreductase that is differentially regulated by light. Plant Mol Biol 47:805–813
Suzuki JY, Bauer CE (1995) A prokaryotic origin for light-dependent chlorophyll biosynthesis of plants. Proc Natl Acad Sci USA. 92:3749–3753
Sytina OA et al (2008) Conformational changes in an ultrafast lightdriven enzyme determine catalytic activity. Nature 456:1001–1004
Sytina OA, Heyes DJ, Hunter CN, Groot ML (2009) Ultrafast catalytic processes and conformational changes in the light-driven enzyme protochlorophyllide oxidoreductase (POR). Biochem Soc Trans 37(Pt 2):387–391
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Townley HE, Sessions RB, Clarke AR, Dafforn TR, Griffiths WT (2001) Protochlorophyllide oxidoreductase: a homology model examined by site-directed mutagenesis. Proteins 44:329–335
von Wettstein D, Gough S, Kannangara CG (1995) Chlorophyll biosynthesis. Plant Cell 7:1039–1057
Wagner D, Przybyla D, Op den Camp R, Kim C, Landgraf F, Lee KP, Würsch M, Laloi C, Nater M, Hideg E, Apel K (2004) The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science 306(5699):1183–1185
Wilks HM, Timko MP (1995) A light-dependent complementation system for analysis of NADPH:protochlorophyllide oxidoreductase: identification and mutagenesis of two conserved residues that are essential for enzyme activity. Proc Natl Acad Sci USA 92:724–728
Yuan M, Zhang DW, Zhang ZW, Chen YE, Yuan S et al (2012) Assembly of NADPH:protochlorophyllide oxidoreductase complex is needed for effective greening of barley seedlings. J Plant Physiol 169:1311–131629
Acknowledgements
We are grateful to P.M. Mullineaux (School of Biological Sciences, University of Essex, UK) for a gift of pGreen and other transformation vectors. We thank K. Kálai and E. Hideg (Institute for Plant Biology, Biological Research Centre, Hungarian Academy of Sciences, Szeged, Hungary) for a gift of the DanePy reagent. We are indebted to Claudia Rossig for help with the construction of the PORB mutant cDNAs and Iga Samol for advice with regard to the seedling viability tests. This work was supported by a Chaire d’Excellence granted to CR by the French Ministry of Research and Education.
Author contributions
FB carried out experiments. AL carried out experiments, AS carried out electron microscopy, SR and DvW analyzed data, CR designed research, carried out experiments analyzed data, StR designed research, carried out research, analyzed data, wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Buhr, F., Lahroussi, A., Springer, A. et al. NADPH:protochlorophyllide oxidoreductase B (PORB) action in Arabidopsis thaliana revisited through transgenic expression of engineered barley PORB mutant proteins. Plant Mol Biol 94, 45–59 (2017). https://doi.org/10.1007/s11103-017-0592-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-017-0592-x