Abstract
Virus infections in grapevine cause important economic losses and affect fruit quality worldwide. Although the phenotypic symptoms associated to viral infections have been described, the molecular plant response triggered by virus infection is still poorly understood in Vitis vinifera. As a first step to understand the fruit changes and mechanisms involved in the compatible grapevine-virus interaction, we analyzed the berry transcriptome in two stages of development in the red wine cultivar Cabernet Sauvignon infected with Grapevine leaf-roll-associated virus-3 (GLRaV-3). Analysis of global gene expression patterns indicate incomplete berry maturation in infected berries as compared to uninfected fruit suggesting viral infection interrupts the normal berry maturation process. Genes with altered expression in berries harvested from GLRaV-3-infected vines as compared to uninfected tissue include anthocyanin biosynthesis and sugar metabolism genes. The reduction in transcript accumulation for sugar and anthocyanin metabolism during fruit development is consistent with a dramatic reduction in anthocyanin biosynthesis as well as reduced sugar levels in berries, a hallmark phenotypic change observed in virus infected grapevines. Analysis of key regulatory factors provides a mechanism for the observed gene expression changes. Our results provide insight into commonly observed phenotypic alterations in virus infected vines and the molecular mechanisms associated with the plant response to the virus during berry ripening.







Similar content being viewed by others
References
Afoufa-Bastien D, Medici A, Jeauffre J, Coutos-Thévenot P, Lemoine R, Atanassova R, Laloi M (2010) The Vitis vinifera sugar transporter gene family: phylogenetic overview and macroarray expression profiling. BMC Plant Biol 10:245
Ascencio-Ibáñez JT, Sozzani R, Lee TJ, Chu TM, Wolfinger RD, Cella R, Hanley-Bowdoin L (2008) Global analysis of Arabidopsis gene expression uncovers a complex array of changes impacting pathogen response and cell cycle during geminivirus infection. Plant Physiol 148:436–454
Atanassova R, Leterrier M, Gaillard C, Agasse A, Sagot E, Coutos-Thevenot P, Delrot S (2003) Sugar-regulated expression of a putative hexose transport gene in grape. Plant Physiol 131:326–334
Babu M, Gagarinova AG, Brandle JE, Wang A (2008) Association of the transcriptional response of soybean plants with soybean mosaic virus systemic infection. J Gen Virol 89:1069–1080
Bell E, Mullet JE (1993) Characterization of an Arabidopsis lipoxygenase gene responsive to methyl jasmonate and wounding. Plant Physiol 103:1133–1137
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 57:289–300
Bernstein E, Caudy AA, Hammond SM, Hannon GJ (2001) Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409:363–366
Blevins T, Rajeswaran R, Shivaprasad PV, Beknazariants D, Si-Ammour A, Park HS, Vazquez F, Robertson D, Meins F Jr, Hohn T, Pooggin MM (2006) Four plant Dicers mediate viral small RNA biogenesis and DNA virus induced silencing. Nucleic Acids Res 34:6233–6246
Borgo M, Angelini E (2002) Influence of grapevine leafroll (GLRaV3) on Merlot cv. grape production. Bulletin OIV 75:611–622
Boss PK, Davies C, Robinson SP (1996) Analysis of the expression of anthocyanin pathway genes in developing Vitis vinifera L. cv Shiraz grape berries and the implications for pathway regulation. Plant Physiol 111:1059–1066
Breitling R, Armengaud P, Amtmann A, Herzyk P (2004) Rank products: a simple, yet powerful, new method to detect differentially regulated genes in replicated microarray experiments. FEBS Lett 573:83–92
Bustin SA (2010) Why the need for qPCR publication guidelines?-The case for MIQE. Methods 50:217–226
Castellarin SD, Matthews MA, Di Gaspero G, Gambetta GA (2007) Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 227:101–112
Cheng NH, Su CL, Carter SA, Nelson RS (2000) Vascular invasion routes and systemic accumulation patterns of tobacco mosaic virus in Nicotiana benthamiana. Plant J 23:349–362
Christie P, Alfenito MR, Walbot V (1994) Impact of low temperature stress on general phenylpropanoid and anthocyanin pathways: enhancement of transcript abundance and anthocyanin pigmentation in maize seedlings. Planta 194:541–549
Christov I, Stefanov D, Velinov T, Goltsev V, Georgieva K, Abracheva P, Genova Y, Christov N (2007) The symptomless leaf infection with grapevine leafroll associated virus 3 in grown in vitro plants as a simple model system for investigation of viral effects on photosynthesis. J Plant Physiol 164:1124–1133
Coetzee B, Freeborough MJ, Maree HJ, Celton JM, Rees DJ, Burger JT (2010) Deep sequencing analysis of viruses infecting grapevines: virome of a vineyard. Virology 400:157–163
Cogotzi L, Giampetruzzi A, Nölke G, Orecchia M, Elicio V, Castellano MA, Martelli GP, Fischer R, Schillberg S, Saldarelli P (2009) An assay for the detection of grapevine leafroll-associated virus 3 using a single-chain fragment variable antibody. Arch Virol 154(1):19–26
Conde C, Agasse A, Glissant D, Tavares R, Geros H, Delrot S (2006) Pathways of glucose regulation of monosaccharide transport in grape cells. Plant Physiol 141:1563–1577
Coombe BG (1992) Research on development and ripening of the grape berry. Am J Enol Vitic 43:101–110
Coombe BG (1995) Adoption of a system for identifiying grapevine growth stages. Aust J Grape Wine Res 1:100–110
Coombe BG, McCarthy MG (2000) Dynamics of grape berry growth and physiology of ripening. Aust J Grape Wine Res 6:131–135
Cui X, Fan B, Scholz J, Chen Z (2007) Roles of Arabidopsis cyclin-dependent kinase C complexes in cauliflower mosaic virus infection, plant growth, and development. Plant Cell 19:1388–1402
da Silva FG, Iandolino A, Al-Kayal F, Bohlmann MC, Cushman MA, Lim H, Ergul A, Figueroa R, Kabuloglu EK, Osborne C, Rowe J, Tattersall E, Leslie A, Xu J, Baek J, Cramer GR, Cushman JC, Cook DR (2005) Characterizing the grape transcriptome. Analysis of expressed sequence tags from multiple Vitis species and development of a compendium of gene expression during berry development. Plant Physiol 139:574–597
Deluc LG, Grimplet J, Wheatley MD, Tillett RL, Quilici DR, Osborne C, Schooley DA, Schlauch KA, Cushman JC, Cramer GR (2007) Transcriptomic and metabolite analyses of Cabernet Sauvignon grape berry development. BMC Genomics 8:429
Deluc LG, Quilici DR, Decendit A, Grimplet J, Wheatley MD, Schlauch KA, Merillon JM, Cushman JC, Cramer GR (2009) Water deficit alters differentially metabolic pathways affecting important flavor and quality traits in grape berries of Cabernet Sauvignon and Chardonnay. BMC Genomics 10:212
Eisen MB, Spellman PT, Brown PO, Botstein D (1998) Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 95:14863–14868
Epstein MA, Holt SJ (1963) Electron microscope observations on the surface adenosine triphosphatase-like enzymes of HeLa cells infected with herpes virus. J Cell Biol 19:337–347
Espinoza C, Medina C, Somerville S, Arce-Johnson P (2007a) Senescence-associated genes induced during compatible viral interactions with grapevine and Arabidopsis. J Exp Bot 58:3197–3212
Espinoza C, Vega A, Medina C, Schlauch K, Cramer G, Arce-Johnson P (2007b) Gene expression associated with compatible viral diseases in grapevine cultivars. Funct Integr Genomics 7:95–110
Fiore N, Prodan S, Montealegre J, Aballay E, Pino AM, Zamorano A (2008) Survey of grapevine viruses in chile. J Plant Pathol 90:125–130
Fotopoulos V, Gilbert MJ, Pittman JK, Marvier AC, Buchanan AJ, Sauer N, Hall JL, Williams LE (2003) The monosaccharide transporter gene, AtSTP4, and the cell-wall invertase, Atbetafruct1, are induced in Arabidopsis during infection with the fungal biotroph Erysiphe cichoracearum. Plant Physiol 132:821–829
Garcia-Rodriguez Pozo, Azcon-Aguilar Ferrol (2005) Expression of a tomato sugar transporter is increased in leaves of mycorrhizal or Phytophthora parasitica-infected plants. Mycorrhiza 15:489–496
Gautier L, Cope L, Bolstad BM, Irizarry RA (2004) affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 20:307–315
Gollop R, Farhi S, Perl A (2001) Regulation of the leucoanthocyanidin dioxygenase gene expression in Vitis vinifera. Plant Sci 161:579–588
Gollop R, Even S, Colova-Tsolova V, Perl A (2002) Expression of the grape dihydroflavonol reductase gene and analysis of its promoter region. J Exp Bot 53:1397–1409
Gosalvez-Bernal B, Genoves A, Navarro JA, Pallas V, Sanchez-Pina MA (2008) Distribution and pathway for phloem-dependent movement of Melon necrotic spot virus in melon plants. Mol Plant Pathol 9:447–461
Grimplet J, Deluc LG, Tillett RL, Wheatley MD, Schlauch KA, Cramer GR, Cushman JC (2007) Tissue-specific mRNA expression profiling in grape berry tissues. BMC Genomics 8:187
Gutha LR, Casassa LF, Harbertson JF, Naidu RA (2010) Modulation of flavonoid biosynthetic pathway genes and anthocyanins due to virus infection in grapevine (Vitis vinifera L.) leaves. BMC Plant Biol 10:187–204
Hayes MA, Davies C, Dry IB (2007) Isolation, functional characterization, and expression analysis of grapevine (Vitis vinifera L.) hexose transporters: differential roles in sink and source tissues. J Exp Bot 58:1985–1997
Hayes MA, Feechan A, Dry IB (2010) Involvement of abscisic acid in the coordinated regulation of a stress-inducible hexose transporter (VvHT5) and a cell wall invertase in grapevine in response to biotrophic fungal infection. Plant Physiol 153:211–221
Hümmer W, Schreier P (2008) Analysis of proanthocyanidins. Mol Nutr Food Res 52:1381–1398
Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4:249–264
Jensen AB, Raventos D, Mundy J (2002) Fusion genetic analysis of jasmonate-signalling mutants in Arabidopsis. Plant J 29:595–606
Johansen DA (1940) Plant microtechnique. Mac Graw Hill, NY, USA, pp 523
Katari MS, Nowicki SD, Aceituno FF, Nero D, Kelfer J, Thompson LP, Cabello JM, Davidson RS, Goldberg AP, Shasha DE, Coruzzi GM, Gutiérrez RA (2010) VirtualPlant: a software platform to support systems biology research. Plant Physiol 152:500–515
Kirik A, Mudgett MB (2009) SOBER1 phospholipase activity suppresses phosphatidic acid accumulation and plant immunity in response to bacterial effector AvrBsT. Proc Natl Acad Sci USA 106:20532–20537
Kobayashi S, Ishimaru M, Hiraoka K, Honda C (2002) Myb-related genes of the Kyoho grape (Vitis labruscana) regulate anthocyanin biosynthesis. Planta 215:924–933
Kobayashi S, Goto-Yamamoto N, Hirochika H (2004) Retrotransposon-induced mutations in grape skin color. Science 304:982
Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, Antonescu C, Salzberg SL (2004) Versatile and open software for comparing large genomes. Genome Biol 5:R12
La Camera S, Balagué C, Göbel C, Geoffroy P, Legrand M, Feussner I, Roby D, Heitz T (2008) The Arabidopsis patatin-like protein 2 (PLP2) plays an essential role in cell death execution and differentially affects biosynthesis of oxylipins and resistance to pathogens. Mol Plant Microbe Interact 22:469–481
Lartey R, Ghoshroy S, Ho J, Citovsky V (1997) Movement and subcellular localization of a tobamovirus in Arabidopsis. Plant J 12:537–545
Ling KS, Zhu HY, Gonsalves D (2004) Complete nucleotide sequence and genome organization of grapevine leafroll-associated virus 3, type member of the genus Ampelovirus. J Gen Virol 85:2099–2102
Lo Piero AR, Puglisi I, Rapisarda P, Petrone G (2005) Anthocyanins accumulation and related gene expression in red orange fruit induced by low temperature storage. J Agric Food Chem 53:9083–9088
Lund ST, Peng FY, Nayar T, Reid KE, Schlosser J (2008) Gene expression analyses in individual grape (Vitis vinifera L.) berries during ripening initiation reveal that pigmentation intensity is a valid indicator of developmental staging within the cluster. Plant Mol Biol 68:301–315
Martelli G (1993) True virus diseases. Leafroll. In: Martelli G (ed) Graft-transmissible diseases of grapevines. Handbook for detection and diagnosis. FAO, Rome, pp 37–44
Martelli G, Boudon-Padieu E (2006) Directory of infectious diseases of grapevines and viroses and virus-like diseases of the grapevine. Options Medit Ser B 55:7–201
Martelli G, Walter B (1998) Virus certification in grapevines. In: Hadidi A, Khetarpal R, Koganezawa H (eds) Plant virus disease control. APS Press, St. Paul, p 684
Matus JT, Vega A, Loyola R, Serrano C, Cabrera S, Arce-Johnson P (2008) Phytoplasma and virus detection in commercial plantings of Vitis vinifera cv. Merlot exhibiting premature berry dehydration. Electron J Biotechnol 11(5):7–8
Matus JT, Loyola R, Vega A, Pena-Neira A, Bordeu E, Arce-Johnson P, Alcalde JA (2009) Post-veraison sunlight exposure induces MYB-mediated transcriptional regulation of anthocyanin and flavonol synthesis in berry skins of Vitis vinifera. J Exp Bot 60:853–867
Mori K, Goto-Yamamoto N, Kitayama M, Hashizume K (2007) Loss of anthocyanins in red-wine grape under high temperature. J Exp Bot 58:1935–1945
Orecchia M, Nölke G, Saldarelli P, Dell’Orco M, Uhde-Holzem K, Sack M, Martelli G, Fischer R, Schillberg S (2008) Generation and characterization of a recombinant antibody fragment that binds to the coat protein of grapevine leafroll-associated virus 3. Arch Virol 153(6):1075–1084
Peña-Neira A, Hernández T, García-Vallejo C, Estrella I, Suarez JA (2000) A survey of phenolic compounds in Spanish wines from different geographical origins. Eur Food Res Technol 210:445–448
Peña-Neira A, Dueñas M, Duarte A, Hernandez T, Estrella I, Loyola E (2004) Effects of ripening stages and of plant vegetative vigor on the phenolic composition of grapes (Vitis vinifera L.) cv. Cabernet Sauvignon in the Maipo Valley (Chile). Vitis 43:51–57
Peña-Neira A, Cáceres A, Pastenes C (2007) Low molecular weight phenolic and anthocyanin composition of grape skins from cv. Syrah (Vitis vinifera L.) in the Maipo Valley (Chile): effect of clusters thinning and vineyard yield. Food Sci Technol Int 13:153–158
Pilati S, Perazzolli M, Malossini A, Cestaro A, Dematte L, Fontana P, Dal Ri A, Viola R, Velasco R, Moser C (2007) Genome-wide transcriptional analysis of grapevine berry ripening reveals a set of genes similarly modulated during three seasons and the occurrence of an oxidative burst at veraison. BMC Genomics 8:428
Pollard KS, Laan vd (2004) Choice of a null distribution in resampling-based multiple testing. J Stat Plan Inference 125:85–100
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212
Rotter A, Camps C, Lohse M, Kappel C, Pilati S, Hren M, Stitt M, Coutos-Thevenot P, Moser C, Usadel B, Delrot S, Gruden K (2009) Gene expression profiling in susceptible interaction of grapevine with its fungal pathogen Eutypa lata: extending MapMan ontology for grapevine. BMC Plant Biol 9:104
Shalitin D, Wolf S (2000) Cucumber mosaic virus infection affects sugar transport in melon plants. Plant Physiol 123:597–604
Shimizu T, Satoh K, Kikuchi S, Omura T (2007) The repression of cell wall- and plastid-related genes and the induction of defenserelated genes in rice plants infected with rice dwarf virus. Mol Plant Microbe Interact 20:247–254
Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P (2006) Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol 140:637–646
Teng S, Keurentjes J, Bentsink L, Koornneef M, Smeekens S (2005) Sucrose-specific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/PAP1 gene. Plant Physiol 139:1840–1852
Terrier N, Glissant D, Grimplet J, Barrieu F, Abbal P, Couture C, Ageorges A, Atanassova R, Leon C, Renaudin JP, Dedaldechamp F, Romieu C, Delrot S, Hamdi S (2005) Isogene specific oligo arrays reveal multifaceted changes in gene expression during grape berry (Vitis vinifera L.) development. Planta 222:832–847
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:1465–6906
Venencie C, Uveira MN, Guiet S (1997) Maturité polyphénolique du raisin mise en place d’une méthode d’analyse de routine. Revue Francaise d’Oenologie 167:36–41
Vignault C, Vachaud M, Cakir B, Glissant D, Dedaldechamp F, Buttner M, Atanassova R, Fleurat-Lessard P, Lemoine R, Delrot S (2005) VvHT1 encodes a monosaccharide transporter expressed in the conducting complex of the grape berry phloem. J Exp Bot 56:1409–1418
Vitrac X, Larronde F, Krisa S, Decendit A, Deffieux G, Merillon JM (2000) Sugar sensing and Ca2+-calmodulin requirement in Vitis vinifera cells producing anthocyanins. Phytochemistry 53:659–665
Walker AR, Lee E, Bogs J, McDavid DA, Thomas MR, Robinson SP (2007) White grapes arose through the mutation of two similar and adjacent regulatory genes. Plant J 49:772–785
Whitham SA, Quan S, Chang HS, Cooper B, Estes B, Zhu T, Wang X, Hou YM (2003) Diverse RNA viruses elicit the expression of common sets of genes in susceptible Arabidopsis thaliana plants. Plant J 33:271–283
Whitham SA, Yang C, Goodin MM (2006) Global impact: elucidating plant responses to viral infection. Mol Plant Microbe Interact 19:1207–1215
Wise RP, Caldo RA, Hong L, Shen L, Cannon EK, Dickerson JA (2007) BarleyBase/PLEXdb: a unified expression profiling database for plants and plant pathogens. In: Edwards D (ed) Plant bioinformatics—methods and protocols. Methods in molecular biology, vol 406. Humana Press, Totowa, NJ, pp 347–363
Yamane T, Jeong ST, Goto-Yamamoto N, Koshita Y, Kobayashi S (2006) Effects of temperature on anthocyanin biosynthesis in grape berry skins. Am J Enol Vitic 57:54–59
Zenoni S, Ferrarini A, Giacomelli E, Xumerle L, Fasoli M, Malerba G, Bellin D, Pezzotti M, Delledonne M (2010) Characterization of transcriptional complexity during berry development in Vitis vinifera using RNA-Seq. Plant Physiol 152:1787–1795
Acknowledgments
This work was supported by CORFO-Innova 07Genoma01, Millennium Nucleus for Plant Functional Genomics (P06-009-F) and FONDECYT 1100709. We are grateful to Dr. Michael Handford (Universidad de Chile) for critically reading the manuscript and assistance in language support. We also thank Hector Morales for his contribution in HPLC-DAD analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
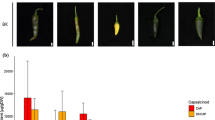
Detection of viral disease in uninfected and GLRaV-3-infected vines. The evaluation of thirteen viruses that have a high infectious incidence in Chile was carried out by RT-PCR amplifying a specific fragment of each virus. The figure shown one example of the detection of GLRaV-3 and GFKV viral disease (A) in Vitis vinifera leaves. A fragment of the virus replicase was amplified (274 bp and 262 bp, respectively). Lane numbers represent different grapevines analyzed. All amplifications were done in similar conditions for the viruses. Transmission electron micrographs of cross-sections of (B) a berry, (C) a virus-infected phloem cell and (D) a healthy virus-free phloem cell. Each micrograph scale is represented with a black line (500 nm) depicted at the bottom of each figure (PPTX 4392 kb)
Figure S2
Hierarchical clustering analysis of grape berries genes showing a differential expression pattern in response to GLRaV-3 during ripening (p < 0.05). The dendrogram and coloured image were produced as described in the Materials and Methods. The color scale ranges from saturated green for log ratios -5.8 and below, to saturated red for log ratios +5.5 and above. Each gene is represented by a single row of colored boxes. A single column represents each experimental point as follows: infected berries at veraison (LR3 V, yellow), uninfected berries at veraison (CV, green) infected ripening berries (LR3M, pink) and uninfected ripening berries (CM, purple). Twelve separate clusters are indicated by colored vertical bars (correlation coefficient < 0.92) (PPTX 1657 kb)
Table S1
Primers designed for virus detection in grapevine plants (DOCX 86 kb)
Table S2
Primers designed for gene expression analysis by RT-qPCR (DOCX 107 kb)
Table S3
Genes affected by ripening in uninfected grape berries (XLS 106 kb)
Table S4
Genes affected by ripening in GLRaV-3 infected grape berries (XLS 74 kb)
Table S5
Genes affected by viral compatible infection in grape berries at veraison (E-L35) (XLS 39 kb)
Table S6
Genes affected by viral compatible infection in grape berries during ripening (EL38) (XLS 70 kb)
Table S7
Physiological analysis of Cabernet Sauvignon berries from uninfected and infected-plants at four berry developmental stages (DOC 31 kb)
Table S8
Concentration of all anthocyanin compounds from uninfected and infected at four berry developmental stages (DOCX 44 kb)
Rights and permissions
About this article
Cite this article
Vega, A., Gutiérrez, R.A., Peña-Neira, A. et al. Compatible GLRaV-3 viral infections affect berry ripening decreasing sugar accumulation and anthocyanin biosynthesis in Vitis vinifera . Plant Mol Biol 77, 261–274 (2011). https://doi.org/10.1007/s11103-011-9807-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-011-9807-8