Abstract
Purpose
Modern research is increasingly focusing on the study of new viruses and the re-emergence of past microbes, such as Coronaviruses, particularly Sars-Cov2 that was responsible for the very recent pandemic.
Methods and Results
This infection manifested itself and still continues to manifest as a severe respiratory syndrome. The main discriminator of whether or not one succeeds in overcoming this infection may depend on a great many factors, but the main one is definitely determined by vaccination, which has minimized hospitalizations and more severe syndromes.
Conclusion
Recently, a new virus, the monkeypox virus, which was previously confined to Central and West Africa but is now gradually spreading to more than 30 countries including the United States of America, where such an infection is not endemic, is coming forward again.
Similar content being viewed by others
The Smallpox
Smallpox viruses are a very large group of DNA viruses that cause infections in both humans and animals. Smallpox originated with the infection of a subspecies of vertebrates namely cows, and their immunity was responsible for the discovery of a vaccine against smallpox in humans, thanks to Edward Jenner, called the "father of immunization" [1]. Smallpox and monkeypox are closely related, and even the symptoms, fever and skin rushes, are strikingly similar. Monkeypox was discovered in 1970 in the Democratic Republic of Congo, but its epidemiology remains "ad horas" unknown. Viruses that usually infect animals and that are thus confined to their own world, very often can evolve and adapt to the environment in a way that allows a "species jump" and thus leading to infection and transmission among humans. This is what happened with Sars-Cov-2 and is what is progressively happening with Monkeypox [2]. This problem is mainly attributable to the promiscuity that very often occurs between the human and animal worlds in underdeveloped countries and, in fact, before the declaration of some cases of monkeypox in non-African countries, such as the United States of America, this infection was endemic only in: Cameroon, Central African Republic, Democratic Republic of Congo, and Nigeria [3]. Smallpox immunization with the vaccine was also effective against monkeypox. However, it is strongly discouraged to carry out such vaccination after 1980 since this infection shares several clinical and pathological factors with other microbial infections, such as Varicella Zooster Virus, cowpox, and many others [4]. The process of total eradication of smallpox worldwide was initiated in 1958 by the World Health Assembly received a report on the catastrophic consequences of smallpox in 63 countries leading then in 1967, under the tutelage of the World Health Organization to a global campaign that led to the eradication of smallpox finally in 1977. On May 8, 1980, the World Health Assembly announced to the entire momdo that smallpox had been eradicated [5, 6].
Monkeypox
The current re-emergence of monkeypox cases may be due both to a decline in vaccination and to a gene development of the virus itself that would then be able to implant itself more easily into the human genome. This should be considered, therefore, an important cue both to continue gene sequencing of the virus by keeping it "under control" and to highlight host preferences and possible hot reservoirs that continue to develop [7].
Monkeypox has a lower incidence of severe forms than COVID-19, although there are many concerns about the geographical spread and further resurgence of the infection [8, 9]. Between 2010 and 2019, a few cases were reported in Nigeria after 40 years of silencing infections. Since 2003, however, cases of monkeypox have also been reported in countries where the disease was not endemic. In particular, there are reports that transmission would have occurred as a result of the importation of rodents from Ghana into the United States. Studies show that the probability of animal-to-human transmission is high [10]. The first cases were probably detected in one of the following countries, Nigeria, the United States, Singapore. [11,12,13], however to date the first epidemic outbreak is still unclear where it occurred. In the United Kingdom, cases of hospital transmission and cases that occurred via the family route have been identified [14]. Epidemiological data show that infected cases can be carried by travelers [15]. In addition, the epidemic potential corresponding to R0 >1 is evidence of transmission between humans [16]. The outbreak of a high proportion of monkeypox cases as of May 2022 with non-directed transmission from endemic countries has raised several issues referring to how this infection could spread, but especially to the fact that the line of transmission may have changed in the meantime. This "mutated" transmission could thus raise serious health issues, which at the dawn of the end of the Coronavirus pandemic, is certainly one of the major issues to be addressed. Even though, compared with COVID-19 pandemic, it is noteworthy that there are already ready-made vaccines as a weapon for MPX, which is a substantial advantage [17, 18].
Biology and Physiopathology
Monkeypox virus (MPXV) belongs to the family Poxviridae [19]. Poxviruses are viruses with a linear double-stranded DNA (dsDNA) genome composed of about 200 genes. Generally, for this type of virus. molecular genetics shows that about half of the genes serve for viral replication, while the remaining half are the so-called accessory genes, which are useful for the virus to penetrate cells into the host organism [20].
The Poxviridae family consists of several viruses, all descended from a rodent probably[21], including cowpox virus (CPXV) camel pox virus (CMLV), and several new variants emerging and identified in recent years [22, 23]. Immunity acquired by infection or vaccination against a virus in this family can also protect against infection of other species. Human monkeypox is typically a zoonosis that clinically resembles smallpox but a lower mortality rate and human-to-human transmission and also differs from smallpox epidemiologically [24, 25].
Biological fluids, respiration, and wound exudate are the main inducer of virus transmission. In addition, the virus is highly stable as demonstrated by laboratory studies [26].
Monkeypoxvirus (MPXV), in spite of its name, is widespread in African rodents especially squirrels, which are now considered the virus' maintenance reservoir [27, 28]. Two strains of MPXV are known today: a more virulent one that can lead to 10% mortality from the Congo Basin, while another milder one is found in West Africa [29]. MPXV can still infect additional animals such as dogs and other rodents with relatively low doses [30,31,32,33,34,35,36]. "Wild" mice are susceptible to MPXV (CAST/eij strain of mice) and differ from the more resistant classical mice [37,38,39,40].
The CAST mouse/MPXV model may have advantages for studying correlations of immunity and vaccine efficacy.
Monkeypox is similar to but milder than the now extinct smallpox. Its manifestation consists of three stages:
-
1.
Incubation: can range from 7 to 14 days, but is generally around 13 days.
-
2.
Prodromal phase: includes fever and lymphadenopathy
-
3.
Skin rash.
The lymphadenopathy characteristic of the prodromal phase is the essential element that distinguishes monkeypox from smallpox and chickenpox.
The rash also deserves a separate characterization: it is in fact distinguished in turn into several phases. An initial macular phase in which the papules appear rosaceous, but flat and not raised. These papules then become denser, vesicular and pustular. They then evolve further to become scabs that will then inevitably fall off. The rash can affect the face, trunk, and extremities, and sometimes the genitals, and all of these areas are involved at the same level, so the manifestation occurs simultaneously in all of the above areas. Extreme care must be taken with papules in the pustular phase, as they contain active virus that by direct transmission can infect another individual [41]. Secondary symptomatology may be of serious concern compared with primary manifestations. This, in fact, can occur with bronchopneumonia, gastroenteritis, sepsis, encephalitis, and keratitis [42] (Table I).
Although how monkeypox manages to maintain itself in the wild is still unknown, in recent decades the research world has been asking ever more probing questions to prevent this same virus from implanting itself in new hosts outside endemic areas. Certainly one of the main reasons why this monkeypox manages to stay active in the wild is that it uses different hosts. One of the most concerning alerts is the implantation of this virus in ground squirrels in regions of North America, which would then represent one of the most dangerous outbreaks in the coming years. [43]. The rationale for why this virus is of concern lies in the fact that it can lurk in any mammalian cell, and the interval at which it maintains itself in the host is variable. Infectivity may depend on several factors, chief among them the responsiveness of the host's immune system. An interesting study considered 528 case infections of Monkeypox diagnosed between April 27 and June 24, 2022, in 16 countries. The study showed that during infection, 95% of people presented with a rash, 73% with anogenital lesions, and 41% with mucosal lesions. Common systemic features occurring preceding, concurrently with, or after the rash included fever (62%), lethargy (41%), myalgia (31%), and headache (27%) [44].
Replication
Antiviral factor K is a protein that inhibits the multiplication of the viral genetic material and, consequently, the multiplication of the virus, thus causing the infection to stop or be attenuated. It is targeted by two viral genes E3L and K3L. It has been found that primate K protein genes have undergone substantial modifications and are susceptible to inhibition of the K3L gene [44, 45]. The main modification that would have originated is that the viral genome has evolved small inhibitors of human antiviral K protein. For these reasons, it is very likely that much more virulent variants could be generated in humans. In fact, the greatest fear is that other types of variants of the same virus family could be generated. Smallpox virus mutates less rapidly than RNA viruses because the DNA genome has a DNA polymerase that has a 3'-5' exonuclease correction activity [46]. The mutation rate of poxviruses could result in up to 2 nucleotide mutations in the genome per year compared to the dozens that can occur in an RNA virus [47,48,49]. Poxviruses therefore vary much less even than SARS-CoV-2 [50] however, it must be said that the smallpox virus genome is large and flexible and allows for large changes to the structure causing loss or increase of genes altering viral phenotypes very rapidly. Generally however, mutants have repeats in their genome of a viral gene that is often the direct target of the drug therapy being undertaken [51, 52] and because of this the virus in order to resist the effect of the drug increases the production of that affected gene.
Vaccine
It is curious to note that the practice used until 1800 for immunization in the East against smallpox was that of "variolation," which consisted of subcutaneous implantation of portions of virus extrapolated from scabs of infected patients. This practice made it possible to avoid the more severe and violent forms of smallpox. Later an English physician Jenner reported a viable alternative. He noticed that the lesions on the hands of milkers who contracted cowpox were similar to those produced by variolation. He then verified whether bovine virus inoculation could avoid the pustules caused by variolation. This finding produced an important assessment: the two viruses, i.e., cowpox and smallpox, are very similar and coming from the same family (Orthopoxvirus) provide cross immunity. The efforts therefore led by WHO to eradicate smallpox ended in 1977 when precisely the last case of smallpox was diagnosed in Somalia. Despite this it should be noted that the vaccine used by Jenner in the beginning was different than the one used in the vaccine campaign. Jenner used cowpox virs (CPXV), while the one used in the vaccine campaign was live vaccinia smallpox virus (VACV). The eradication of smallpox was a formidable, but more importantly encouraging achievement that saved millions of lives and also allowed for the discontinuation of vaccination since it was estimated that vaccination protects for an interval of 20 to 30 years [53].
This remained silent until the first decade of the 2000s when following the Twin Towers terrorist attack, fearing that it might be the prelude to a bioterrorist attack, the United States immediately worked to develop both a vaccine and a pool of antiviral drugs. Currently, two antiviral drugs and two smallpox vaccines are on the market and approved by the FDA that could also have positive efficacy in treating monkeypox virus. The first new generation vaccine was ACAM2000, SIMILAR TO Dryvax because it was obtained on a cell culture with a clone of Dryvax. Vaccines such as the latter generate immunity with antibodies and B cells detected even 60 years later and specific immunity against human monkeypox virus as high as 85 percent [54].
ACAM2000 contains replication competent VACV and is administered by skin scarification. ACAM2000 is contraindicated in pregnancy and in cases of established immunodeficiency and appears to have a risk/benefit profile similar to Dryvax for which the most noted side effect is myopericarditis. The second vaccine synthesized but not yet approved for human use is MVA-BN. It is produced with the modified Ankara vaccine strain (MVA). MVA is altered in replication in most mammalian cells, in part due to the loss of two host genes. It is administered with a double dose of subcutaneous injection 4 weeks apart [55]. The safety profile is extremely encouraging, especially the efficacy shown in studies from animal trials tested for smallpox virus and monkeypox virus. In July 2022, the European Medicines Agency's Committee for Medicinal Products also recommended extending IMNAVEX use in the European Union, for immunization of adults against monkeypox [56]. To confirm this efficacy, the EU will collect data resulting from an observational study conducted during the monkeypox epidemic.
Antiviral Drugs
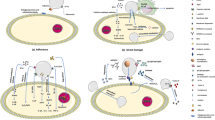
Tecovirimat and Brincidofovir are the two drugs to date approved by the FDA for monkeypox. Tecovirimat acts on the virus envelope by preventing virion release and blocking the activity of the VP37 protein, encoded by a gene that is present throughout the orthopoxvirus genus family [57]. Blocking the interaction of VP37 inhibits the activation of the Rab9 GTPase and TIP47 cells, thus preventing the replication of fully functional cells, capable of exiting the cell and spreading the virus long distances passing from cell to cell. In contrast, the second drug is the propharmaceutical of the antiviral cidofovir [58]. There is lipid conjugation with cidofovir, which thus allows this drug to be used at lower concentrations, consequently reducing its toxicity and still allowing it to have a targeted action on inhibition of viable DNA replication. Brincidofovir is already on the market for the treatment of cytomegalovirus. Limited studies of drug use in some cases of monkeypox have shown that tecovirimat is more effective than brincidofovir because the latter can develop pharmacoresistance with mutations in F13L (highly conserved viral membrane protein) and E9L (DNA polymerase) [59, 60]. On July 13, 2018, tecovirimat has been authorized for the placing on the American market as the first drug indicated for the treatment of smallpox, to which the indication was later extended to also treat MPX. Afterwards, Tecovirimat has been also authorized for the placing on the European market on 06 January 2022 [61]. In the context of the monkeypox outbreak, EU government has already been facilitating the conduct of large multinational trials in the EU on the use of the antiviral tecovirimat by reviewing the trial protocols and liaising with the Clinical Trial Coordination Group (CTCG) and national regulatory bodies to coordinate and facilitate the approval of clinical trial applications by national competent authorities. Recent evidence has shown that several interesting studies are underway that are identifying new compounds with antiviral activity against the virus responsible for Monkeypox monkeypox. Tyrosine kinase inhibitors of the Src/Abl family are conceivable as potential inhibitors of viral release from the cell [62]. The methylene blue derivative, PAV-866, has shown in vitro antiviral activity against various Orthopoxviruses. including monkeypox virus by blocking virus binding, fusion, and entry [63]. In order to limit as much as possible a new scenario that could harken back to the recent pandemic from which we may be getting out of, the biology of this virus certainly needs to be studied in depth to try to assess any genetic changes while keeping them under control and limiting transmission to humans as much as possible Fig. 1.
Expert Opinion
Since the beginning of the monkeypox epidemic and until October 4, 2022, there have been approximately 20,000 confirmed cases of monkeypox (MPX) in EU/EEA countries. Spain, France, and Germany are the European countries that have reported the largest cases of infection. The Clade II b variant appears to be responsible for the current epidemic by giving clinically less severe symptomatology with less transmissibility and lower mortality. The person's immune response generates the individual's degree of response to the disease. However, it appears that vaccines on the market for smallpox eradication could provide some immunity against MPX as well. The vaccines approved to date against MPX are recommended only for certain high-risk persons, and there is no universal vaccination. Much confusion still exists about the intradermal or subcutaneous route of administration in different countries, so the important thing today is to ensure the right doses to guarantee immunization. However, the intradermal route, with a lower dose than the subcutaneous route has limited data in the literature to date about its efficacy. In addition, not all countries are aligned on the population categories recommended for smallpox vaccination. To date, there is no approved treatment specifically for Monkeypox infections. However, some antivirals developed for use in smallpox patients may prove useful against MPX. These antivirals include: tecovirimat or ST-246 (TPOXX); brincidofovir (Tembexa); and cidofovir (Vistide). Additionally, intravenous vaccinia immune globulin (VIGIV), which is licensed for the treatment of complications from smallpox (vaccinia) vaccination, may be authorized for use to treat monkeypox and other pox viruses during an outbreak. Clinical evidence on efficacy against MPX is needed for antiviral agents; to date there are few results demonstrating antiviral activity against orthopoxvirus. In addition, no data are available on the efficacy of VIGIV in the treatment of monkeypox virus infection. The use of VIGIV has no proven benefit in the treatment of monkeypox, and it is not known whether a person with severe monkeypox infection would benefit from treatment with VIGIV. Cidofovir, brincidofovir in the treatment of monkeypox cases in people have no supporting clinical evidence, only data of antiviral activity on orthopoxvirus. Well-organized clinical trials are needed to generate the right evidence.
Conclusion
The epidemic caused by monkeypox unlike COVID-19 is not having the same severity and speed of spread, both because of the different biological characteristics of the virus and the ready availability of different vaccines and antiviral agents for smallpox and monkeypox. The rapid initiation of infection control measures and the use of vaccines and antiviral agents are important strategies for controlling the monkeypox epidemic. To limit as much as possible a new scenario that could resemble the recent pandemic from which we may be about to emerge, the biology of this virus certainly needs to be studied in depth to try to assess any genetic changes while keeping them under control and limiting transmission to humans as much as possible. Regardless, the new reality is that human monkeypox is no longer a rare zoonotic disease and needs more public health attention.
Data Availability
Full availability of data and materials. All stated data can be provided on request to the reader.
References
Pal M, Mengstie F, Kandi V. Epidemiology, diagnosis, and control of monkeypox disease: a comprehensive review. Am J Infect Dis Microbiol. 2017;5:94–9.
Chakraborty C, Bhattacharya M, Nandi SS, Mohapatra RK, Dhama K, Agoramoorthy G. Appearance and re-appearance of zoonotic disease during the pandemic period: long-term monitoring and analysis of zoonosis is crucial to confirm the animal origin of SARS-CoV-2 and monkeypox virus. Vet Q. 2022;42:119–24. https://doi.org/10.1080/01652176.2022.2086718.
Multi-country monkeypox outbreak in non-endemic countries. (2022). Accessed: June 20, 2022.
Sironi VA, Inglese S, Lavazza A. The "one health" approach in the face of COVID-19: how radical should it be?. Philos Ethics Humanit Med. 2022, 17. https://doi.org/10.1186/s13010-022-00116-2
Alakunle E, Moens U, Nchinda G, Okeke MI. Monkeypox virus in Nigeria: infection biology, epidemiology, and evolution. Viruses. 2020:12. https://doi.org/10.3390/v12111257.
World Health Organization. The Global Eradication of Smallpox: Final Report of the Global Commission for the Certification of Smallpox Eradication. Geneva: World Health Organization; 1980. Global Commission for Certification of Smallpox Eradication. [Google Scholar]
Riedel S. Edward Jenner and the history of smallpox and vaccination. Proc (Bayl Univ Med Cent). 2005 Jan;18(1):21–5. https://doi.org/10.1080/08998280.2005.11928028.
Vitiello A, La Porta R, Trama U, et al. Pandemic COVID-19, an update of current status and new therapeutic strategies. Naunyn-Schmiedeberg's Archives of Pharmacology. 2022;395(10):1159–65.
Ferrara F, Zovi A, Trama U, Vitiello A. Nirmatrelvir–remdesivir association for non-hospitalized adults with COVID-19, point of view. Inflammopharmacology. 2022;30(5):1927–31.
Niles EG, Seto J. Vaccinia virus gene D8 encodes a virion transmembrane protein. J Virol. 1988;62:3772–8.
Erez N, Achdout H, Milrot E, Schwartz Y, Wiener-Well Y, Paran N, et al. Diagnosis of imported monkeypox, Israel, 2018. Emerg Infect Dis. 2019;25(5):980–3. https://doi.org/10.3201/eid2505.190076.
Vaughan A, Aarons E, Astbury J, Balasegaram S, Beadsworth M, Beck CR, et al. Two cases of monkeypox imported to the United Kingdom, September 2018. Euro Surveill. 2018;23(38).
Monkeypox—United Kingdom of Great Britain and Northern Ireland (Nigeria). 11 June 2021.
World Health Organization. Monkeypox—United Kingdom of Great Britain and Northern Ireland. 8 July 2021.
Nigeria Centre for Disease Control. Monkeypox Situation Report in Nigeria. May 31, 2021.
Grant R, Nguyen LL, Breban R. Modelling human-to-human transmission of monkeypox. Bull World Health Organ. 2020;98(9):638–40. https://doi.org/10.2471/BLT.19.242347.
Vitiello A, Porta RL, Pianesi L, Ferrara F. COVID-19 pandemic: vaccine and new monoclonal antibodies, point of view. Irish J Med Sci. 2022;191(1):487–8.
Vitiello A, Ferrara F, Zovi A, Trama U, Boccellino M. Pregnancy and COVID-19, focus on vaccine and pharmacological treatment. J Reproductive Immunol. 2022;151:103630.
Moss B. Poxviridae. In: Knipe DM, Howley PM, editor. Fields virology, Vol. 2. 6th ed. Philadelphia: Lippincott Williams and Wilkins; 2013. p. 2129–2159.
Senkevich TG, Yutin N, Wolf YI. Ancient gene capture and recent gene loss shape the evolution of orthopoxvirus-host interaction genes. MBio. 2021;12(4):e0149521.
Damon IK. Poxviruses. In: Knipe DM, Howley PM, editors. Fields virology. Vol. 2. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2013. p. 2160–2184. [Google Scholar]ues caused by possibly novel orthopoxvirus, Italy. Emerg Infect Dis. 2017;23(12):1941–1949.
Gao J, Gigante C, Khmaladze E. Genome sequences of akhmeta virus, an early divergent old world orthopoxvirus. Viruses. 2018;10(5):252.
Breman JG, Kalisa R, Steniowski MV, Zanotto E, Gromyko AI, Arita I. Human monkeypox, 1970–79. Bull World Health Organ. 1980;58:165–82.
Parker S, et al. Mousepox in the C57BL/6 strain provides an improved model for evaluating anti-poxvirus therapies. Virology. 2009;385:11–21.
Wolff HL, Croon JJ. The survival of smallpox virus (variola minor) in natural circumstances. Bull World Health Organ. 1968;38(3):492–3.
Khodakevich L, et al. The role of squirrels in sustaining monkeypox virus transmission. Trop Geogr Med. 1987;39:115–22.
Reynolds MG, et al. A silent enzootic of an orthopoxvirus in Ghana, West Africa: Evidence for multi-species involvement in the absence of widespread human disease. Amer J Tropical Med Hyg. 2010;82:746–54.
Tesh RB, Watts DM, Sbrana E, Siirin M, Popov VL, Xiao SY. Experimental infection of ground squirrels (Spermophilius tridecemlineatus) with Monkeypox virus. Emerging Infect Dis. 2004;10:1563–7.
Sbrana E, Xiao SY, Newman PC, Tesh RB. Comparative pathology of North American and central African strains of monkeypox virus in a ground squirrel model of the disease. Amer J Trop Med Hyg. 2007;76:155–64.
Guarner J, et al. Monkeypox transmission and pathogenesis in prairie dogs. Emerging Infect Dis. 2004;10:426–31.
Langohr IM, Stevenson GW, Thacker HL, Regnery RL. Extensive lesions of Monkeypox in a prairie dog (Cynomys sp). Vet Pathol. 2004;41:702–7.
Xiao SY, Sbrana E, Watts DM, Siirin M, Travassos da Rosa APA. Experimental infection of prairie dogs with monkeypox virus. Emerging Inf Dis. 2005;11:539–45.
Hutson CL, et al. A prairie dog animal model of systemic orthopoxvirus disease using West African and Congo Basin strains of monkeypox virus. J Gen Virol. 2009;90:323–33.
Schultz DA, Sagartz JE, Huso DL, Buller RML. Experimental infection of an African dormouse (Graphiurus kelleni) with monkeypox virus. Virology. 2009;383:86–92.
Fenner F. The eradication of smallpox. Prog Med Virol. 1977;23:1–21.
Hanna W, Baxby D. Studies in smallpox and vaccination. Rev Med Virol. 1913;2002(12):201–9.
Hutson CL, et al. Comparison of West African and Congo Basin monkeypox viruses in BALB/c and C57BL/6 mice. PLoS ONE. 2010;5:e8912.
Stabenow J, Buller RM, Schriewer J, West C, Sagartz JE, Parker S. A mouse model of lethal infection for evaluating prophylactics and therapeutics against monkeypox virus. J Virol. 2010;84:3909–20.
Osorio JE, Iams KP, Meteyer CU, Rocke TE. Comparison of monkeypox viruses pathogenesis in mice by in vivo imaging. PLoS ONE. 2009;4:e6592.
Americo JL, Moss B, Earl PL. Identification of wild-derived inbred mouse strains highly susceptible to monkeypox virus infection for use as small animal models. J Virol. 2010;84:8172–80.
McCollum AM, Damon IK. Human monkeypox. Clin Infect Dis. 2014 Jan;58(2):260–7.
Ogoina D, Iroezindu M, James HI, et al. Clinical course and outcome of human monkeypox in Nigeria. Clin Infect Dis. 2020;71(8):e210–4.
Reynolds MG, Doty JB, McCollum AM, et al. Monkeypox re-emergence in Africa: a call to expand the concept and practice of One health. Expert Rev Anti Infect Ther. 2019;17(2):129–39.
Thornhill JP, Barkati S, Walmsley S, Rockstroh J, Antinori A, Harrison LB, Palich R, Nori A, Reeves I, Habibi MS, Apea V, Boesecke C, Vandekerckhove L, Yakubovsky M, Sendagorta E, Blanco JL, Florence E, Moschese D, Maltez FM, et al. SHARE-net Clinical Group. Monkeypox Virus Infection in Humans across 16 Countries - April-June 2022. N Engl J Med. 2022;387(8):679–91. https://doi.org/10.1056/NEJMoa2207323.
Elde NC, Child SJ, Geballe AP, et al. Protein kinase R reveals an evolutionary model for defeating viral mimicry. Nature. 2009;457(7228):485–9.
Rothenburg S, Seo EJ, Gibbs JS, et al. Rapid evolution of protein kinase PKR alters sensitivity to viral inhibitors. Nat Struct Mol Biol. 2009 Jan;16(1):63–70.
Moss B. Poxvirus DNA replication. Cold Spring Harbor Perspect Biol. 2013;5(9):a010199.
Firth C, Kitchen A, Shapiro B, et al. Using time-structured data to estimate evolutionary rates of double-stranded DNA viruses. Mol Biol Evol. 2010;27(9):2038–51.
Duggan AT, Perdomo MF, Piombino-Mascali D, et al. 17 th century variola virus reveals the recent history of smallpox. Curr Biol. 2016;26(24):3407–12.
Duffy S, Shackelton LA, Holmes EC. Rates of evolutionary change in viruses: patterns and determinants. Nat Rev Genet. 2008 Apr;9(4):267–76.
Ghafari M, du Plessis L, Raghwani J, et al. Purifying selection determines the short-term time dependency of Evolutionary Rates in SARS-CoV-2 and pH1N1 influenza. Mol Biol Evol. 2022;39(2):msac009.
Slabaugh MB, Roseman NA, Mathews CK. Amplification of the ribonucleotide reductase small subunit gene: analysis of novel joints and the mechanism of gene duplication in vaccinia virus. Nucleic Acids Res. 1989;17(17):7073–88.
Erlandson KJ, Cotter CA, Charity JC, et al. Duplication of the A17L locus of vaccinia virus provides an alternate route to rifampin resistance. J Virol. 2014;88(19):11576–85.
Hammarlund E, Lewis MW, Hansen SG, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9(9):1131–7.
Crotty S, Felgner P, Davies H, et al. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171(10):4969–73.
Fine PE, Jezek Z, Grab B, et al. The transmission potential of monkeypox virus in human populations. Int J Epidemiol. 1988 Sep;17(3):643–50.
European Medicines Agency. https://www.ema.europa.eu/en/news/ema-response-monkeypox-public-health-emergency. July 27, 2022.
Food and Drug Administration. https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-indication-treatment-smallpox. July 13, 2018.
Food and Drug Administration. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-drug-treat-smallpox. April 6, 2021.
Duraffour S, Lorenzo MM, Zöller G, et al. ST-246 is a key antiviral to inhibit the viral F13L phospholipase, one of the essential proteins for orthopoxvirus wrapping. J Antimicrob Chemother. 2015;70(5):1367–80.
Italian Medicines Agency. https://www.aifa.gov.it/documents/20142/1621464/2022.07.25_com_EMA_raccomandazione_Imvanex_vaiolo_scimmie_IT.pdf . July 22, 2022.
Reeves PM, Smith SK, Olson VA, et al. Variola and monkeypox viruses utilize conserved mechanisms of virion motility and release that depend on Abl and Src family tyrosine kinases. J Virol. 2011;85(1):21–31. https://doi.org/10.1128/jvi.01814-10.
Priyamvada L, Burgado J, Baker-Wagner M, et al. New methylene blue derivatives suggest novel anti-orthopoxviral strategies. Antiviral Res. 2021;191:105086. https://doi.org/10.1016/j.antiviral.2021.105086.
Code Availability
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Not applicable
Consent to participate
Not applicable
Consent to publish
The authors consent to the publication of the manuscript
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare that the opinions expressed are of a personal nature and do not in any way commit the responsibility of the Administrations to which they belong.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zovi, A., Ferrara, F., Sorrentino, S. et al. What Do We Know About the Smallpox Virus? A Journey Between Clinic and Therapy. Pharm Res 40, 459–465 (2023). https://doi.org/10.1007/s11095-022-03447-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-022-03447-z