Abstract
Purpose
The purpose of this study was to evaluate the in vitro lung dissolution of amorphous and crystalline powder formulations of rifampicin in polyethylene oxide (PEO) and 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine (DPPC), and to predict the in vivo plasma concentration–time profiles using the in vitro data.
Methods
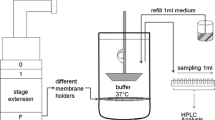
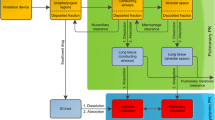
The in vitro dissolution and permeation profiles of respirable rifampicin particles were studied using a custom-made dissolution apparatus. Data from the in vitro dissolution test were used to estimate the parameters to be used as the input for the simulation of in vivo plasma concentration–time profiles using STELLA® software. For prediction of in vivo profiles, a one-compartment model either with a first order elimination or with a Michaelis–Menten kinetics-based elimination was used.
Results
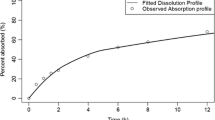
Compared to the crystalline formulation, the amorphous formulation showed rapid in vitro dissolution suggesting their possible faster in vivo absorption and higher plasma concentrations of rifampicin following lung delivery. However, the simulations suggested that both powder formulations would result in similar plasma-concentration time profiles of rifampicin.
Conclusions
Use of an in vitro dissolution test coupled with a simulation model for prediction of plasma-concentration time profiles of an inhaled drug was demonstrated in this work. These models can also be used in the design of inhaled formulations by controlling their release and dissolution properties to achieve desired lung retention or systemic absorption following delivery to the lungs.










Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Forbes B, Richer NH, Buttini F. Dissolution: a critical performance characteristic of inhaled products. In: Pulmonary Drug Delivery: Advances and Challenges. Wiley & Sons; 2015. p. 223–40.
Riley T, Christopher D, Arp J, Casazza A, Colombani A, Cooper A, et al. Challenges with Developing In Vitro Dissolution Tests for Orally Inhaled Products (OIPs). AAPS PharmSciTech [Internet]. 2012;13(3):978–89. Available from: https://doi.org/10.1208/s12249-012-9822-3.
Velaga SP, Djuris J, Cvijic S, Rozou S, Russo P, Colombo G, et al. Dry powder inhalers: An overview of the in vitro dissolution methodologies and their correlation with the biopharmaceutical aspects of the drug products. Eur J Pharm Sci [Internet]. 2018;113:18–28. Available from: https://www.sciencedirect.com/science/article/pii/S0928098717304980.
Floroiu A, Klein M, Krämer J, Lehr C-M. Towards standardized dissolution techniques for in vitro performance testing of dry powder inhalers. Dissolut Technol. 2018;25:6–18.
Gerde P, Malmlöf M, Havsborn L, Sjöberg C-O, Ewing P, Eirefelt S, et al. Dissolv It: an in vitro method for simulating the dissolution and absorption of inhaled dry powder drugs in the lungs. Assay Drug Dev Technol. 2017;15(2):77–88.
Fröhlich E. Toxicity of orally inhaled drug formulations at the alveolar barrier: parameters for initial biological screening. Drug Deliv. 2017;24(1):891–905.
Radivojev S, Zellnitz S, Paudel A, Froehlich E. Searching for physiologically relevant in vitro dissolution techniques for orally inhaled drugs. Int J Pharm. 2019;556:45–56.
Hochhaus G, Horhota S, Hendeles L, Suarez S, Rebello J. Pharmacokinetics of orally inhaled drug products. AAPS J. 2015;17(3):769–75.
García-Arieta A. A European perspective on orally inhaled products: in vitro requirements for a biowaiver. J Aerosol Med Pulm Drug Deliv. 2014;27(6):419–29.
Rohrschneider M, Bhagwat S, Krampe R, Michler V, Breitkreutz J, Hochhaus G. Evaluation of the transwell system for characterization of dissolution behavior of inhalation drugs: effects of membrane and surfactant. Mol Pharm. 2015;12(8):2618–24.
Borghardt JM, Weber B, Staab A, Kloft C. Pharmacometric models for characterizing the pharmacokinetics of orally inhaled drugs. AAPS J. 2015;17(4):853–70.
Hassoun M, Malmlöf M, Scheibelhofer O, Kumar A, Bansal S, Selg E, et al. Use of PBPK modeling to evaluate the performance of dissolv it, a biorelevant dissolution assay for orally inhaled drug products. Mol Pharm. 2019;16(3):1245–54.
Bhagwat S, Schilling U, Chen M-J, Wei X, Delvadia R, Absar M, et al. Predicting pulmonary pharmacokinetics from in vitro properties of dry powder inhalers. Pharm Res. 2017;34(12):2541–56.
Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56(6):588–99.
Campbell EA, Korzheva N, Mustaev A, Murakami K, Nair S, Goldfarb A, et al. Structural mechanism for rifampicin inhibition of bacterial RNA polymerase. Cell. 2001;104(6):901–12.
Maggi N, Pasqualucci CR, Ballotta R, Sensi P. Rifampicin: a new orally active rifamycin. Chemotherapy. 1966;11(5):285–92.
Misra A, Hickey AJ, Rossi C, Borchard G, Terada H, Makino K, et al. Inhaled drug therapy for treatment of tuberculosis. Tuberculosis. 2011;91(1):71–81.
Traini D, Young PM. Drug delivery for tuberculosis: is inhaled therapy the key to success? Vol. 8, Therapeutic delivery. Future Science; 2017. p. 819–21.
Dartois V. The path of anti-tuberculosis drugs: from blood to lesions to mycobacterial cells. Nat Rev Microbiol. 2014;12(3):159–67.
Pelizza G, Nebuloni M, Ferrari P, Gallo GG. Polymorphism of rifampicin. Farmaco Sci. 1977;32(7):471–81.
Henwood SQ, Liebenberg W, Tiedt LR, Lötter AP, De Villiers MM. Characterization of the solubility and dissolution properties of several new rifampicin polymorphs, solvates, and hydrates. Drug Dev Ind Pharm. 2001;27(10):1017–30.
Groom CR, Bruno IJ, Lightfoot MP, Ward SC. The Cambridge structural database. Acta Crystallogr Sect B. International Union of Crystallography; 2016;72:171–9.
Nogueira L de PP, de Oliveira YS, Fonseca J de C, Costa WS, Raffin FN, Ellena J, et al. Crystalline structure of the marketed form of Rifampicin: a case of conformational and charge transfer polymorphism. J Mol Struct. 2018;1155:260–6.
Agrawal S, Ashokraj Y, Bharatam PV, Pillai O, Panchagnula R. Solid-state characterization of rifampicin samples and its biopharmaceutic relevance. Eur J Pharm Sci. 2004;22(2–3):127–44.
Kostewicz ES, Aarons L, Bergstrand M, Bolger MB, Galetin A, Hatley O, et al. PBPK models for the prediction of in vivo performance of oral dosage forms. Eur J Pharm Sci. 2014;57:300–21.
Eedara BB, Tucker IG, Das SC. A STELLA simulation model for in vitro dissolution testing of respirable size particles. Sci Rep. 2019;9(1):1–14.
Chakraborty S, Yadav L, Aggarwal D. Prediction of in vivo drug performance using in vitro dissolution coupled with STELLA: a study with selected drug products. Drug Dev Ind Pharm. 2015;41(10):1667–73.
Eedara BB, Tucker IG, Das SC. In vitro dissolution testing of respirable size anti-tubercular drug particles using a small volume dissolution apparatus. Int J Pharm. 2019;559:235–44.
Eriksson J, Thörn H, Sjögren E, Holmstén L, Rubin K, Lennernäs H. Pulmonary Dissolution of Poorly Soluble Compounds Studied in an ex Vivo Rat Lung Model. Mol Pharm [Internet]. 2019 Jul 1;16(7):3053–64. Available from: https://doi.org/10.1021/acs.molpharmaceut.9b00289.
Khadka P, Hill PC, Zhang B, Katare R, Dummer J, Das SC. A study on polymorphic forms of rifampicin for inhaled high dose delivery in tuberculosis treatment. Int J Pharm. 2020;587: 119602.
Kim CS, Eldridge MA. Aerosol deposition in the airway model with excessive mucus secretions. J Appl Physiol. 1985;59(6):1766–72.
Shah S, Fung K, Brim S, Rubin BK. AnIn Vitro Evaluation of the Effectiveness of Endotracheal Suction Catheters. Chest. 2005;128(5):3699–704.
Costa P, Lobo JMS. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13(2):123–33.
Garcia Contreras L, Sung J, Ibrahim M, Elbert K, Edwards D, Hickey A. Pharmacokinetics of inhaled rifampicin porous particles for tuberculosis treatment: insight into rifampicin absorption from the lungs of guinea pigs. Mol Pharm. 2015;12(8):2642–50.
Khadka P, Sinha S, Tucker IG, Dummer J, Hill PC, Katare R, et al. Pharmacokinetics of rifampicin after repeated intra-tracheal administration of amorphous and crystalline powder formulations to Sprague Dawley rats. Eur J Pharm Biopharm. 2021;162:1–11.
Oberdörster G. Lung clearance of inhaled insoluble and soluble particles. J Aerosol Med. 1988;1(4):289–330.
Murdande SB, Pikal MJ, Shanker RM, Bogner RH. Aqueous solubility of crystalline and amorphous drugs: challenges in measurement. Pharm Dev Technol. 2011;16(3):187–200.
British Pharmacopoeia. Appendix XII C: Consistency of formulated preparations. Her Majesty’s Stationary Office, London; 2012.
Svensson RJ, Aarnoutse RE, Diacon AH, Dawson R, Gillespie SH, Boeree MJ, et al. A population pharmacokinetic model incorporating saturable pharmacokinetics and autoinduction for high rifampicin doses. Clin Pharmacol Ther. 2018;103(4):674–83.
Acocella G. Clinical pharmacokinetics of rifampicin. Clin Pharmacokinet. 1978;3(2):108–27.
Laperche Y, Graillot C, Arondel J, Berthelot P. Uptake of rifampicin by isolated rat liver cells. Interaction with sulfobromophthalein uptake and evidence for separate carriers. Biochem Pharmacol. 1979;28(13):2065–9.
Son Y-J, McConville JT. Development of a standardized dissolution test method for inhaled pharmaceutical formulations. Int J Pharm. 2009;382(1–2):15–22.
Zhang H, Fan Q, Wang YE, Neal CR, Zuo YY. Comparative study of clinical pulmonary surfactants using atomic force microscopy. Biochim Biophys Acta (BBA)-Biomembranes. 2011;1808(7):1832–42.
Notter RH, Chess PR, Pryhuber GS. Lung surfactant: overview. In: Janes SM, editor. Encycl Respir Med. Second. Oxford: Academic Press; 2022. p. 90–9.
Acknowledgements
The authors would also like to acknowledge Nicole Wood for her help in running the in vitro dissolution experiments.
Author information
Authors and Affiliations
Contributions
Conceptualization: Prakash Khadka, Ian G. Tucker, Shyamal Das.
Methodology: Prakash Khadka, Ian G. Tucker, Shyamal Das.
Data curation: Prakash Khadka.
Formal analysis: Prakash Khadka, Ian G. Tucker, Shyamal Das.
Writing, review and editing: Prakash Khadka, Ian G. Tucker, Shyamal Das.
Project administration: Shyamal Das.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khadka, P., Tucker, I.G. & Das, S.C. In vitro Dissolution Testing of Rifampicin Powder Formulations For Prediction of Plasma Concentration–Time Profiles After Inhaled Delivery. Pharm Res 40, 1153–1163 (2023). https://doi.org/10.1007/s11095-022-03439-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-022-03439-z