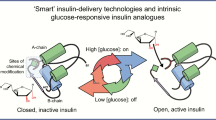
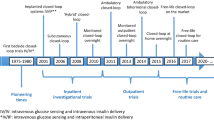
Abstract
The development of new diabetes treatment strategies has garnered much interest given that conventional management therapies for type 1 diabetes fail to provide optimal glycemic control while creating a high burden of self-care to patients. Stimuli-responsive, “closed-loop” systems are particularly attractive due to their ability to mimic dynamic ß cell function by releasing insulin in response to fluctuating glucose levels in real-time and with minimal patient discomfort. In this short review, we focus on stimuli-responsive, reservoir-based insulin delivery devices. We explore and evaluate systems that are either physiologically or externally triggered. While obstacles remain before such technologies can be translated to clinical settings, further optimization of delivery systems forebodes that these technologies will have a tremendous impact on type 1 diabetes treatment.






Similar content being viewed by others
References
Federation ID. International diabetes federation 2020 [Available from: https://idf.org/.
Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383(9911):69–82.
Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–22.
Ravaine V, Ancla C, Catargi B. Chemically controlled closed-loop insulin delivery. J Control Release. 2008;132(1):2–11.
Nijhoff MF, de Koning EJP. Artificial pancreas or novel Beta-cell replacement therapies: a race for optimal glycemic control? Curr Diab Rep. 2018;18(11):110.
Lachin J, Mcgee P, Palmer J, Grp DER, Grp DER. Impact of C-peptide preservation on metabolic and clinical outcomes in the diabetes control and complications trial. Diabetes. 2014;63(2):739–48.
Shapiro A, Lakey J, Ryan E, Korbutt G, Toth E, Warnock G, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–8.
Ernst A, Bowers D, Wang L, Shariati K, Plesser M, Brown N, et al. Nanotechnology in cell replacement therapies for type 1 diabetes. Adv Drug Deliv Rev. 2019;139:116–38.
Mo R, Jiang T, Di J, Tai W, Gu Z. Emerging micro- and nanotechnology based synthetic approaches for insulin delivery. Chem Soc Rev. 2014;43(10):3595–629.
Wang J, Wang Z, Yu J, Kahkoska AR, Buse JB, Gu Z. Glucose-Responsive Insulin and Delivery Systems: Innovation and Translation. Adv Mater. 2019:e1902004.
Langer R. Advances in drug delivery. Cancer Res. 2017;77.
Yu J, Zhang Y, Yan J, Kahkoska AR, Gu Z. Advances in bioresponsive closed-loop drug delivery systems. Int J Pharm. 2018;544(2):350–7.
Wilson R, Turner A. Glucose-oxidase - an ideal enzyme. Biosens Bioelectron. 1992;7(3):165–85.
Bankar S, Bule M, Singhal R, Ananthanarayan L. Glucose oxidase - an overview. Biotechnol Adv. 2009;27(4):489–501.
Gordijo C, Koulajian K, Shuhendler A, Bonifacio L, Huang H, Chiang S, et al. Nanotechnology-enabled closed loop insulin delivery device: in vitro and in vivo evaluation of glucose-regulated insulin release for diabetes control. Adv Funct Mater. 2011;21(1):73–82.
Gordijo C, Shuhendler A, Wu X. Glucose-responsive bioinorganic Nanohybrid membrane for self-regulated insulin release. Adv Funct Mater. 2010;20(9):1404–12.
Chu M, Chen J, Gordijo C, Chiang S, Ivovic A, Koulajian K, et al. In vitro and in vivo testing of glucose-responsive insulin-delivery microdevices in diabetic rats. Lab Chip. 2012;12(14):2533–9.
Diez P, de Avila B, Ramirez-Herrera D, Villalonga R, Wang J. Biomedical nanomotors: efficient glucose-mediated insulin release. Nanoscale. 2017;9(38):14307–11.
Ortiz-Prado E, Dunn JF, Vasconez J, Castillo D, Viscor G. Partial pressure of oxygen in the human body: a general review. Am J Blood Res. 2019;9(1):1–14.
Klumb L, Horbett T. Design of insulin delivery devices based on glucose sensitive membranes. J Control Release. 1992;18(1):59–80.
Yin R, Bai M, He J, Nie J, Zhang W. Concanavalin A-sugar affinity based system: binding interactions, principle of glucose-responsiveness, and modulated insulin release for diabetes care. Int J Biol Macromol. 2019;124:724–32.
Wu S, Huang X, Du X. Glucose- and pH-responsive controlled release of cargo from protein-gated carbohydrate-functionalized Mesoporous silica Nanocontainers. Angewandte Chemie-International Edition. 2013;52(21):5580–4.
Powell AE, Leon MA. Reversible interaction of human lymphocytes with the mitogen concanavalin a. Exp Cell Res. 1970;62(2):315–25.
Kataoka K, Hisamitsu I, Sayama N, Okano T, Sakurai Y. Novel sensing system for glucose based on the complex formation between phenylborate and fluorescent diol compounds. J Biochem. 1995;117(6):1145–7.
Matsumoto A, Tanaka M, Matsumoto H, Ochi K, Moro-oka Y, Kuwata H, et al. Synthetic "smart gel" provides glucose-responsive insulin delivery in diabetic mice. Science Advances. 2017;3(11).
Matsumoto A, Ishii T, Nishida J, Matsumoto H, Kataoka K, Miyahara Y. A synthetic approach toward a self-regulated insulin delivery system. Angewandte Chemie-International Edition. 2012;51(9):2124–8.
Matsumoto A, Ikeda S, Harada A, Kataoka K. Glucose-responsive polymer bearing a novel phenylborate derivative as a glucose-sensing moiety operating at physiological pH conditions. Biomacromolecules. 2003;4(5):1410–6.
Blum A, Kammeyer J, Rush A, Callmann C, Hahn M, Gianneschi N. Stimuli-responsive Nanomaterials for biomedical applications. J Am Chem Soc. 2015;137(6):2140–54.
Prausnitz M, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26(11):1261–8.
Sirsi S, Borden M. State-of-the-art materials for ultrasound-triggered drug delivery. Adv Drug Deliv Rev. 2014;72:3–14.
Smith N, Lee S, Maione E, Roy R, McElligott S, Shung K. Ultrasound-mediated transdermal transport of insulin in vitro through human skin using novel transducer designs. Ultrasound Med Biol. 2003;29(2):311–7.
Smith N, Lee S, Shung K. Ultrasound-mediated transdermal in vivo transport of insulin with low-profile cymbal arrays. Ultrasound Med Biol. 2003;29(8):1205–10.
Lee S, Snyder B, Newnham RE, Smith NB. Noninvasive ultrasonic transdermal insulin delivery in rabbits using the light-weight cymbal array. Diabetes Technol Ther. 2004;6(6):808–15.
Park EJ, Werner J, Smith NB. Ultrasound mediated transdermal insulin delivery in pigs using a lightweight transducer. Pharm Res. 2007;24(7):1396–401.
Park E-J, Werner J, Jaiswal D, Smith NB, editors. Closed-Loop controlled noninvasive ultrasonic glucose sensing and insulin delivery. American Institute of Physics; 2010.
Jabbari N, Asghari MH, Ahmadian H, Mikaili P. Developing a commercial air ultrasonic ceramic transducer to transdermal insulin delivery. J Med Signals Sens. 2015;5(2):117–22.
Timko BP, Arruebo M, Shankarappa SA, McAlvin JB, Okonkwo OS, Mizrahi B, et al. Near-infrared-actuated devices for remotely controlled drug delivery. Proc Natl Acad Sci U S A. 2014;111(4):1349–54.
Zhang Y, Yu J, Kahkoska AR, Wang J, Buse JB, Gu Z. Advances in transdermal insulin delivery. Adv Drug Deliv Rev. 2019;139:51–70.
Roxhed N, Samel B, Nordquist L, Griss P, Stemme G. Painless drug delivery through microneedle-based transdermal patches featuring active infusion. IEEE Trans Biomed Eng. 2008;55(3):1063–71.
Kochba E, Levin Y, Raz I, Cahn A. Improved insulin pharmacokinetics using a novel microneedle device for intradermal delivery in patients with type 2 diabetes. Diabetes Technol Ther. 2016;18(9):525–31.
ACKNOWLEDGMENTS AND DISCLOSURES
This work was partially supported by the National Science Foundation (SNM-1530522), Juvenile Diabetes Research Foundation (JDRF), the Hartwell Foundation, the National Institutes of Health (NIH, 1R01DK105967-01A1) and the Novo Nordisk Company. The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Meng Deng and Shihuan Kuang
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fuchs, S., Shariati, K. & Ma, M. Stimuli-Responsive Insulin Delivery Devices. Pharm Res 37, 202 (2020). https://doi.org/10.1007/s11095-020-02918-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02918-5