Abstract
Purpose
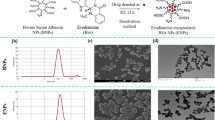
Combinatorial approach can be beneficial for cancer treatment with better patient recovery. Co-delivery of natural and synthetic anticancer drug not only valuable to achieve better anticancer effectivity but also to ascertain toxicity. This study was aimed to co-deliver berberine (natural origin) and doxorubicin (synthetic origin) utilizing conjugation/encapsulation strategy through poly (lactic-co-glycolic acid) (PLGA) nanoparticles.
Methods
Doxorubicin was efficiently conjugated to PLGA via carbodiimide chemistry and the PLGA-doxorubicin conjugate (PDC) was used for encapsulation of berberine (PDBNP).
Results
Significant anti-proliferative against MDA-MB-231 and T47D breast cancer cell lines were observed with IC50 of 1.94 ± 0.22 and 1.02 ± 0.36 μM, which was significantly better than both the bio-actives (p < 0.05). The ROS study revealed that the PDBNP portrayed the slight increase in the reactive oxygen species (ROS) pattern in MDA-MB-231 cell line in a dose-dependent manner, while in T47D cells, no significant change in ROS was seen. PDBNP exhibits significant alteration (depolarization) in mitochondrial membrane permeability and arrest of cell cycle progression at sub G1 phase while the Annexin V/PI assay followed by confocal microscopy resulted into cell death mode to be because of necrosis against MDA-MB-231 cells. In vivo studies in Sprague Dawley rats revealed almost 14-fold increase in half life and a significant increase in plasma drug concentration.
Conclusion
The overall approach of PLGA based co-delivery of doxorubicin and berberine witnessed synergetic effect and reduced toxicity as evidenced by preliminary toxicity studies.












Similar content being viewed by others
Abbreviations
- AFM:
-
Atomic force microscopy
- DCM:
-
Dichloromethane
- EDC:
-
1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide
- hPBMCs:
-
Human Peripheral Blood Mononuclear Cells
- NHS:
-
N-hydroxy succinimide
- PDBNP:
-
Berberine loaded PLGA-doxorubicin nanoparticles
- PDC:
-
PLGA-doxorubicin conjugate
- PLGA:
-
Poly lactide-co-glycolide
- PNP:
-
Blank nanoparticle
- PVA:
-
Polyvinyl alcohol
- RBCs:
-
Red blood cells
- ROS:
-
Reactive oxygen species
- SEM:
-
Scanning electron microscopy
References
Shim G, Kim MG, Kim D, Park JY, Oh YK. Nanoformulation-based sequential combination cancer therapy. Adv Drug Deliv Rev. 2017;115:57–81.
Hu Q, Sun W, Wang C, Gu Z. Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv Drug Deliv Rev. 2016;98:19–34.
Gautam CS, Saha L. Fixed dose drug combinations (FDCs): rational or irrational: a view point. Br J Clin Pharmacol. 2008;65:795–6.
Ducreux M, Malka D, Mendiboure J, Etienne PL, Texereau P, Auby D, et al. Sequential versus combination chemotherapy for the treatment of advanced colorectal cancer (FFCD 2000-05): an open-label, randomised, phase 3 trial. Lancet Oncol. 2011;12:1032–44.
Parhi P, Mohanty C, Sahoo SK. Nanotechnology-based combinational drug delivery: an emerging approach for cancer therapy. Drug Discov Today. 2012;17:1044–52.
Qi SS, Sun JH, Yu HH, Yu SQ. Co-delivery nanoparticles of anti-cancer drugs for improving chemotherapy efficacy. Drug Delivery. 2017;24:1909–26.
Kemp JA, Shim MS, Heo CY, Kwon YJ. “Combo” nanomedicine: co-delivery of multi-modal therapeutics for efficient, targeted, and safe cancer therapy. Adv Drug Deliv Rev. 2016;98:3–18.
Kleibl Z, Kristensen VN. Women at high risk of breast cancer: molecular characteristics, clinical presentation and management. Breast. 2016;28:136–44.
Nabholtz JM, Falkson C, Campos D, Szanto J, Martin M, Chan S, et al. Docetaxel and doxorubicin compared with doxorubicin and cyclophosphamide as first-line chemotherapy for metastatic breast cancer: results of a randomized, multicenter, phase III trial. J Clin Oncol. 2003;21:968–75.
Henderson IC, Berry DA, Demetri GD, Goldstein LJ, Martino S, Ingle JN, et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–83.
Burger H, Foekens JA, Look MP, Meijer-van Gelder ME, Klijn JG, Wiemer EA, et al. RNA expression of breast cancer resistance protein, lung resistance related protein, multi-drug resistance gene-1 in breast cancer: correlation with chemotherapeutic response. Clin Cancer Res. 2003;9:827–36.
Anitha A, Maya S, Deepa N, Chennazhi KP, Naira SV, Tamura H, et al. Efficient water-soluble O-carboxymethyl chitosan nanocarrier for the delivery of curcumin to cancer cells. Carbohydr Polym. 2011;83:452–61.
Murugan C, Rayappan K, Thangam R, Bhanumathi R, Shanthi K, Vivek R, et al. Combinatorial nanocarrier based drug delivery approach for amalgamation of anti-tumor agents in breast cancer cells: an improved nanomedicine strategy. Sci Rep. 2016;6:34053.
Gupta L, Sharma AK, Gothwal A, Khan MS, Khinchi MP, Qayum A, et al. Dendrimer encapsulated and conjugated delivery of berberine: a novel approach mitigating toxicity and improving in vivo pharmacokinetics. Int J Pharm. 2017;528:88–99.
Ebrahimian M, Taghavi S, Ghoreishi M, Sedghi S, Farzad SA, Ramezani M, et al. Evaluation of efficiency of modified polypropylenimine (PPI) with alkyl chains as non-viral vectors used in co-delivery of doxorubicin and TRAILplasmid. AAPS Pharm Sci Tech. 2017;19:31029–36.
Han Y, Zhang P, Chen Y, Sun J, Kong F. Co-delivery of plasmid DNA and doxorubicin by solid lipid nanoparticles for lung cancer therapy. Int J Mol Med. 2014;34(1):191–6.
Khan I, Gothwal A, Sharma AK, Kesharwani P, Gupta L, Iyer AK, et al. PLGA nanoparticles and their versatile role in anticancer drug delivery. Cri Rev Ther Drug Carr Sys. 2016;33(2):159–93.
Khan I, Gothwal A, Sharma AK, Qayum A, Singh SK, Gupta U. Biodegradable nano-architectural PEGylated approach for the improved stability and anticancer efficacy of bendamustine. Int J Bio Macromol. 2016;92:1242–51.
Desgouilles S, Vauthier C, Bazile D, Vacus J, Grossiord JL, Veillard M, et al. The design of nanoparticles obtained by solvent evaporation: a comprehensive study. Langmuir. 2003;19(22):9504–10.
Malvern. ISO13320 2009. Particle Size Analysis - Laser Diffraction Methods, Part 1: General Principles.
Pamunuwa G, Karunaratne V, Karunaratne DN. Effect of lipid composition on in vitro release and skin deposition of curcumin encapsulated liposomes. J Nanomaterials. 2016:1–9.
Ahmad A, Fauzia E, Kumar M, Kumar R. Gelatin-coated Polycaprolactone nanoparticle-mediated Naringenin delivery rescue human mesenchymal stem cells from oxygen glucose deprivation-induced inflammatory stress. ACS Biomater Sci Eng. 2019;5(2):683–95.
Kumar H, Gothwal A, Khan I, Nakhate KT, Alexander A. Ajazuddin, et al. galactose anchored gelatin nanoparticles for primaquine delivery and improved pharmacokinetics: a biodegradable and safe approach for effective anti-plasmodial activity against P. falciparum 3D7 and in vivo hepatocytes targeting. Mol Pharm. 2017;14:3356–69.
Chin DL, Lum BL, Sikic BI, et al. Rapid determination of PEGylated liposomal doxorubicin and its major metabolite in human plasma by ultraviolet-visible high-performance liquid chromatography. J Chromato B. 2002;779:259–69.
Tsai PL, Tsai TH. HPLC determination of berberine in medicinal herbs and a related traditional chinese medicine. Analytical Lett. 2002;35(15):2459–70.
Agarwal GU, Jain NK. Glycoconjugated peptide dendrimers-based nanoparticulate system for the delivery of chloroquine phosphate. Biomaterials. 2007;28:3349–59.
Singhai AK, Jain S, Jain NK. Evaluation of an aqueous injection of Ketoprofen. Pharmazie. 1997;52:149–51.
Jain AK, Thanki K, Jain S. Co-encapsulation of tamoxifen and quercetin in polymeric nanoparticles: implications on oral bioavailability, antitumor efficacy, and drug-induced toxicity. Mol Pharm. 2013;10:3459–74.
Yoo HS, Lee KH, Oh JE, Park TG. In vitro and in vivo anti-tumor activities of nanoparticles based on doxorubicin-PLGA conjugates. J Control Release. 2000;68:419–31.
Wanga X, Wanga Q, Liua Z, Zheng X. Preparation, pharmacokinetics and tumour-suppressive activity of berberine liposomes. J Pharm Pharmacol. 2017;69:625–32.
Fan X, Wang X, Cao M, Wang C, Hu Z, Wu Y-L, et al. “Y”-shape armed amphiphilic star-like copolymers: design, synthesis and dual-responsive unimolecular micelle formation for controlled drug delivery. Polym Chem. 2017;8:5611–20.
Langdon SP. Cancer cell culture, methods and protocols, methods in molecular medicine, Humana Press, 2004.
Meerloo JV, Kaspers GL, Cloos J. Cell sensitivity assays: the MTT assay. Cancer Cell Culture. 2011:237–45.
Maksimenkoa A, Dosiob F, Mougina J, Ferrerob A, Wacka S, Reddya LH, et al. A unique squalenoylated and nonpegylated doxorubicin nanomedicine with systemic long-circulating properties. Proc Natl Acad Sci. 2014;111(2):E217–26.
Cheng J, Teply BA, Sherifi I, Sung J, Luther G, Gu FX. Formulation of functionalized PLGA-PEG nanoparticles for in vivo targeted drug delivery. Biomaterials. 2007;28:869–76.
Diogo CV, Machado NG, Barbosa IA, Serafim TL, Burgeiro A, Paulo J. Berberine as a promising safe anti-Cancer agent-is there a role for mitochondria? Oliveira. Curr Drug Targets. 2011;12:850–9.
Schneckenburger H, Stock K, Lyttek M, Strauss WS, Sailer R. Fluorescence lifetime imaging (FLIM) of rhodamine 123 in living cells. Photochem Photobiol Sci. 2004;3:127–31.
Borodina VM, Zelenin AV. Fluorescence microscopy demonstration of mitochondria in tissue culture cells using berberine. Tsitologiia. 1977;19:1067–8.
Pereira CV, Machado NG, Oliveira PO. Mechanisms of Berberine (natural yellow 18)-induced mitochondrial dysfunction: interaction with the adenine nucleotide translocator. Toxicological Sci. 2008;105(2):408–17.
Lüpertz R, Wätjen W, Kahl R, Chovolou Y. Dose and time-dependent effects of doxorubicin on cytotoxicity, cell cycle and apoptotic cell death in human colon cancer cells. Toxicology. 2010;271:115–21.
Mahmoudi M, Azadmanesh K, Shokrgozar MA, Journeay WS, Laurent S. Effect of nanoparticles on the cell life cycle. Chem Rev. 2011;111:3407–32.
ACKNOWLEDGMENTS AND DISCLOSURES
The authors would like to acknowledge the financial support received from Department of Science and Technology and University Grants Commission, New Delhi, India to Dr. Umesh Gupta in the form of DST Start up Research Grant (for Young Scientists). The first author (IK) also would like to acknowledge Indian Council of Medical Research (ICMR), New Delhi (Award letter no. 45/12/2018-Nan/BMS) for providing Senior Research Fellowship (SRF). GJ thanks CSIR, New Delhi (Grant no. 05/1051(0011)/2018-EMR-I) for providing SRF. The authors declare no competing financial interest. The authors would also like to acknowledge the Central Instrumentation Laboraory Central University of Punjab for extending facilities to carry out microscopy.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 694 kb)
Rights and permissions
About this article
Cite this article
Khan, I., Joshi, G., Nakhate, K.T. et al. Nano-Co-Delivery of Berberine and Anticancer Drug Using PLGA Nanoparticles: Exploration of Better Anticancer Activity and In Vivo Kinetics. Pharm Res 36, 149 (2019). https://doi.org/10.1007/s11095-019-2677-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-019-2677-5