Abstract
Purpose
KRAS is the most frequently mutated gene in human cancers. Despite its direct involvement in malignancy and intensive effort, direct inhibition of KRAS via pharmacological inhibitors has been challenging. RNAi induced knockdown using siRNAs against mutant KRAS alleles offers a promising tool for selective therapeutic silencing in KRAS-mutant lung cancers. However, the major bottleneck for clinical translation is the lack of efficient biocompatible siRNA carrier systems.
Methods
Bovine serum albumin (BSA) nanoparticles were prepared by desolvation method to deliver siRNA targeting the KRAS G12S mutation. The BSA nanoparticles were characterized with respect to their size, zeta potential, encapsulation efficiency and nucleic acid release. Nanoparticle uptake, cellular distribution of nucleic acids, cytotoxicity and gene knock down to interfere with cancer hallmarks, uncontrolled proliferation and migration, were evaluated in KRAS G12S mutant A459 cells, a lung adenocarcinoma cell line.
Results
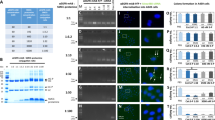
BSA nanoparticles loaded with siRNA resulted in nanoparticles smaller than 200 nm in diameter and negative zeta potentials, displaying optimal characteristics for in vivo application. Encapsulating and protecting the siRNA payload well, the nanoparticles enabled transport to A549 cells in vitro, could evade endosomal entrapment and mediated significant sequence-specific KRAS knockdown, resulting in reduced cell growth of siRNA transfected lung cancer cells.
Conclusions
BSA nanoparticles loaded with mutant specific siRNA are a promising therapeutic approach for KRAS-mutant cancers.







Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30.
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–94.
Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359(13):1367–80.
Dogan S, Shen R, Ang DC, Johnson ML, D'Angelo SP, Paik PK, et al. Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: higher susceptibility of women to smoking-related KRAS-mutant cancers. Clin Cancer Res. 2012;18(22):6169–77.
El Osta B, Behera M, Kim S, Berry LD, Sica G, Pillai RN, et al. Characteristics and outcomes of patients with metastatic KRAS-mutant lung adenocarcinomas: the lung Cancer mutation consortium experience. J Thorac Oncol. 2019.
Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer. 2003;3(6):459–65.
Scheffzek K, Ahmadian MR, Kabsch W, Wiesmuller L, Lautwein A, Schmitz F, et al. The Ras-RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science. 1997;277(5324):333–8.
Merkel OM, Zheng M, Debus H, Kissel T. Pulmonary gene delivery using polymeric nonviral vectors. Bioconjug Chem. 2012;23(1):3–20.
Shepherd FA, Domerg C, Hainaut P, Janne PA, Pignon JP, Graziano S, et al. Pooled analysis of the prognostic and predictive effects of KRAS mutation status and KRAS mutation subtype in early-stage resected non-small-cell lung cancer in four trials of adjuvant chemotherapy. J Clin Oncol. 2013;31(17):2173–81.
Sotillo R, Schvartzman JM, Socci ND, Benezra R. Mad2-induced chromosome instability leads to lung tumour relapse after oncogene withdrawal. Nature. 2010;464(7287):436–40.
Kandil R, Merkel M. Therapeutic delivery of RNA effectors: diseases affecting the respiratory system. Pharmazie. 2016;71(1):21–6.
Merkel OM, Kissel T. Nonviral pulmonary delivery of siRNA. Acc Chem Res. 2012;45(7):961–70.
Tarhini M, Benlyamani I, Hamdani S, Agusti G, Fessi H, Greige-Gerges H, et al. Protein-based nanoparticle preparation via nanoprecipitation method. Materials (Basel). 2018;11(3).
Elzoghby AO, Samy WM, Elgindy NA. Protein-based nanocarriers as promising drug and gene delivery systems. J Control Release. 2012;161(1):38–49.
Elzoghby AO, Samy WM, Elgindy NA. Albumin-based nanoparticles as potential controlled release drug delivery systems. J Control Release. 2012;157(2):168–82.
Gong J, Chen M, Zheng Y, Wang S, Wang Y. Polymeric micelles drug delivery system in oncology. J Control Release. 2012;159(3):312–23.
Tong R, Yala L, Fan TM, Cheng J. The formulation of aptamer-coated paclitaxel-polylactide nanoconjugates and their targeting to cancer cells. Biomaterials. 2010;31(11):3043–53.
Kratz F, Beyer U. Serum proteins as drug carriers of anticancer agents: a review. Drug Deliv. 1998;5(4):281–99.
Kratz F. Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. J Control Release. 2008;132(3):171–83.
Monsigny M, Roche AC, Midoux P. Uptake of neoglycoproteins via membrane lectin(s) of L1210 cells evidenced by quantitative flow cytofluorometry and drug targeting. Biol Cell. 1984;51(2):187–96.
Mo Y, Barnett ME, Takemoto D, Davidson H, Kompella UB. Human serum albumin nanoparticles for efficient delivery of Cu, Zn superoxide dismutase gene. Mol Vis. 2007;13:746–57.
Langer K, Balthasar S, Vogel V, Dinauer N, von Briesen H, Schubert D. Optimization of the preparation process for human serum albumin (HSA) nanoparticles. Int J Pharm. 2003;257(1–2):169–80.
Wartlick H, Spankuch-Schmitt B, Strebhardt K, Kreuter J, Langer K. Tumour cell delivery of antisense oligonuclceotides by human serum albumin nanoparticles. J Control Release. 2004;96(3):483–95.
Feldmann DP, Cheng Y, Kandil R, Xie Y, Mohammadi M, Harz H, et al. In vitro and in vivo delivery of siRNA via VIPER polymer system to lung cells. J Control Release. 2018;276:50–8.
Andima M, Costabile G, Isert L, Ndakala AJ, Derese S, Merkel OM. Evaluation of beta-Sitosterol loaded PLGA and PEG-PLA nanoparticles for effective treatment of breast Cancer: preparation, physicochemical characterization, and antitumor activity. Pharmaceutics. 2018;10(4).
Mehta A, Cordero J, Dobersch S, Romero-Olmedo AJ, Savai R, Bodner J, et al. Non-invasive lung cancer diagnosis by detection of GATA6 and NKX2-1 isoforms in exhaled breath condensate. EMBO Mol Med. 2016;8(12):1380–9.
Wacker M. Nanocarriers for intravenous injection--the long hard road to the market. Int J Pharm. 2013;457(1):50–62.
Jhaveri AM, Torchilin VP. Multifunctional polymeric micelles for delivery of drugs and siRNA. Front Pharmacol. 2014;5:77.
Nichols JW, Bae YH. EPR: evidence and fallacy. J Control Release. 2014;190:451–64.
Danhier F. To exploit the tumor microenvironment: since the EPR effect fails in the clinic, what is the future of nanomedicine? J Control Release. 2016;244(Pt A:108–21.
Park K. Albumin: a versatile carrier for drug delivery. J Control Release. 2012;157(1):3.
Gradishar WJ. Albumin-bound paclitaxel: a next-generation taxane. Expert Opin Pharmacother. 2006;7(8):1041–53.
Fu Q, Sun J, Zhang W, Sui X, Yan Z, He Z. Nanoparticle albumin-bound (NAB) technology is a promising method for anti-cancer drug delivery. Recent Pat Anticancer Drug Discov. 2009;4(3):262–72.
Langiu M, Dadparvar M, Kreuter J, Ruonala MO. Human serum albumin-based nanoparticle-mediated in vitro gene delivery. PLoS One. 2014;9(9):e107603.
Perepelyuk M, Shoyele O, Birbe R, Thangavel C, Liu Y, Den RB, et al. siRNA-encapsulated hybrid nanoparticles target mutant K-ras and inhibit metastatic tumor burden in a mouse model of lung Cancer. Mol Ther Nucleic Acids. 2017;6:259–68.
Zorde Khvalevsky E, Gabai R, Rachmut IH, Horwitz E, Brunschwig Z, Orbach A, et al. Mutant KRAS is a druggable target for pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110(51):20723–8.
Torti D, Trusolino L. Oncogene addiction as a foundational rationale for targeted anti-cancer therapy: promises and perils. EMBO Mol Med. 2011;3(11):623–36.
Acknowledgments and Disclosures
This work was in part supported by the Deutsche Gesellschaft für Muskelkranke e.V. (Grant Number 2017-Me7/1) and ERC Starting Grant ERC-2014-StG – 637,830 “Novel Asthma Therapy” to Olivia Merkel as well as the LMU Excellent Nachwuchsförderungsfonds to Aditi Mehta.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Guest Editor: Joshua Reineke
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Mehta, A., Dalle Vedove, E., Isert, L. et al. Targeting KRAS Mutant Lung Cancer Cells with siRNA-Loaded Bovine Serum Albumin Nanoparticles. Pharm Res 36, 133 (2019). https://doi.org/10.1007/s11095-019-2665-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-019-2665-9