Abstract
Purpose
To study spatial heterogeneity in phase composition when mannitol is co-lyophilized with non-crystallizing lyoprotectant, such as sucrose or trehalose. To study the influence of formulation composition and processing conditions on the extent of mannitol hemihydrate (MHH) formation in the final lyophile.
Methods
We used synchrotron X-ray diffractometry (XRD) to spatially map and thereby comprehensively characterize mannitol phase composition in unperturbed lyophiles. Low temperature thermal analysis and XRD was used to study phase behavior of frozen systems.
Result
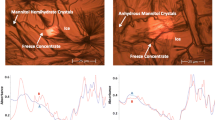
When colyophilized with sucrose, trehalose or lysozyme as a second solute, mannitol crystallized partially as MHH (mannitol hemihydrate). The MHH content, based on the intensity of characteristic MHH peak (d-spacing 4.92 Å), was highest in the middle region of lyophile. This heterogeneity, studied in detail in presence of sucrose, occurred irrespective of cosolute content. Annealing the frozen solution at −30°C for 2 h essentially eliminated the heterogeneity, accompanied by an overall increase in MHH content. From differential scanning calorimetry it was evident that annealing caused mannitol crystallization while XRD revealed the crystallizing phase to be MHH.
Conclusion
The intra-vial heterogeneity and total MHH content in the final lyophile is a complex interplay of formulation composition and processing conditions.

Figure depicting spatial heterogeneity in mannitol hemihydrate content, when mannitol is lyophilized with a cosolute, such as sucrose, trealose or lysozyme.













Similar content being viewed by others
Abbreviations
- API:
-
Active pharmaceutical ingredient
- MHH:
-
Mannitol hemihydrate
- RT:
-
Room temperature
- XRD:
-
X-ray diffractometry
References
Akers M. Parentral preparations. In: Felton L, editor. Remingt. Essentials Pharm. London: The Pharmaceutical Press; 2012. p. 495–533.
Kasper JC, Friess W. The freezing step in lyophilization: Physico-chemical fundamentals, freezing methods and consequences on process performance and quality attributes of biopharmaceuticals. Eur J Pharm Biopharm. 2011;78:248–63.
Liu J. Physical characterization of pharmaceutical formulations in frozen and freeze-dried solid states: techniques and applications in freeze-drying development. Pharm Dev Technol. 2006;11:3–28.
Randolph TW, Searles JA. Freezing and annealing phenomena in lyophilization: effect upon primary drying rate, morphology, and heterogeneity. Am Pharm Rev. 2002;5:40–7.
Liu J, Viverette T, Virgin M, Anderson M, Dalal P. A study of the impact of freezing on the lyophilization of a concentrated formulation with a high fill depth. Pharm Dev Technol. 2005;10:261–72.
Nakagawa K, Hottot A, Vessot S, Andrieu J. Influence of controlled nucleation by ultrasounds on ice morphology of frozen formulations for pharmaceutical proteins freeze-drying. Chem Eng Process Process Intensif. 2006;45:783–91.
Patapoff TW, Overcashier DE. The importance of freezing on Lyophilization cycle development. BioPharm. 2002;15:16–21.
Oddone I, Van Bockstal PJ, De Beer T, Pisano R. Impact of vacuum-induced surface freezing on inter- and intra-vial heterogeneity. Eur J Pharm Biopharm. 2016;103:167–78.
Pikal MJ, Shah S. Intravial distribution of moisture during the secondary drying stage of freeze drying. PDA J Pharm Sci Technol. 1997;51:17–24.
Nakagawa K, Murakami W, Hatanaka T. Redistribution of protein biological activity in a freeze-dried cake. Dry Technol. 2013;31:102–11.
Nail SL, Jiang S, Chongprasert S, Knopp SA. Fundamentals of freeze-drying. Pharm Biotechnol. 2002;14:281–360.
Kim AI, Akers MJ, Nail SL. The physical state of mannitol after freeze-drying: effects of mannitol concentration, freezing rate, and a noncrystallizing cosolute. J Pharm Sci. 1998;87:931–5.
Lueckel B, Bodmer D, Helk B, Leuenberger H. Formulations of sugars with amino acids or mannitol - influence of concentration ratio on the properties of the freeze-concentrate and the lyophilizate. Pharm Dev Technol. 1998;3:325–36.
Cavatur RK, Vemuri NM, Pyne A, Chrzan Z, Toledo-Velasquez D, Suryanarayanan R. Crystallization behavior of mannitol in frozen aqueous solutions. Pharm Res. 2002;19:894–900.
Grohganz H, Lee YY, Rantanen J, Yang M. The influence of lysozyme on mannitol polymorphism in freeze-dried and spray-dried formulations depends on the selection of the drying process. Int J Pharm. 2013;447:224–30.
Cannon AJ, Trappler EH. The influence of lyophilization on the polymorphic behavior of mannitol. PDA J Pharm Sci Technol. 2000;54:13–22.
Izutsu K, Yoshioka S, Terao T. Decreased protein-stabilizing effects of cryoprotectants due to crystallization. Pharm Res. 1993;10:1232–7.
Yu L, Milton N, Groleau EG, Mishra DS, Vansickle RE. Existence of a mannitol hydrate during freeze-drying and practical implications. J Pharm Sci. 1999;88:196–8.
Mehta M, Bhardwaj SP, Suryanarayanan R. Controlling the physical form of mannitol in freeze-dried systems. Eur J Pharm Biopharm. 2013;85:207–13.
Srinivasan JM, Wegiel LA, Hardwick LM, Nail SL. The influence of mannitol hemihydrate on the secondary drying dynamics of a protein formulation: a case study. J Pharm Sci. 2017;106:3583–90.
Cao W, Xie Y, Krishnan S, Lin H, Ricci M. Influence of process conditions on the crystallization and transition of metastable mannitol forms in protein formulations during lyophilization. Pharm Res. 2013;30:131–9.
Chongprasert S, Griesser UJ, Bottorff AT, Williams NA, Byrn SR, Nail SL. Effects of freeze-dry processing conditions on the crystallization of pentamidine isethionate. J Pharm Sci. 1998;87:1155–60.
Hawe A, Frieß W. Impact of freezing procedure and annealing on the physico-chemical properties and the formation of mannitol hydrate in mannitol-sucrose-NaCl formulations. Eur J Pharm Biopharm. 2006;64:316–25.
Liao X, Krishnamurthy R, Suryanarayanan R. Influence of processing conditions on the physical state of mannitol--implications in freeze-drying. Pharm Res. 2007;24:370–6.
Nakagawa K, Murakami W, Andrieu J, Vessot S. Freezing step controls the mannitol phase composition heterogeneity. Chem Eng Res Des. 2009;87:1017–27.
Német Z, Sztatisz J, Demeter Á. Polymorph transitions of bicalutamide: a remarkable example of mechanical activation. J Pharm Sci. 2008;97:3222–32.
Courbion G, Ferey G. Na2Ca3Al2F14: a new example of a structure with “independent F-”-a new method of comparison between fluorides and oxides of different formula. J Solid State Chem. 1988;76:426–31.
Toby BH, Von Dreele RB. GSAS-II: the genesis of a modern open-source all purpose crystallography software package. J Appl Crystallogr. 2013;46:544–9.
Burger A, Henck JO, Hetz S, Rollinger JM, Weissnicht AA, Stöttner H. Energy/temperature diagram and compression behavior of the polymorphs of D-mannitol. J Pharm Sci. 2000;89:457–68.
Johnson RE, Kirchhoff CF, Gaud HT. Mannitol–sucrose mixtures—versatile formulations for protein lyophilization. J Pharm Sci. 2002;91:914–22.
Izutsu K, Kojima S. Excipient crystallinity and its protein-structure-stabilizing effect during freeze-drying. J Pharm Pharmacol. 2002;54:1033–9.
Larsen H, Trnka H, Grohganz H. Formation of mannitol hemihydrate in freeze-dried proteinformulations—a design of experiment approach. Int J Pharm. 2014;460:45–52.
Her LM, Nail SL. Measurement of glass transition temperatures of freeze-concentrated solutes by differential scanning calorimetry. Pharm Res. 1994;11:54–9.
Liao X, Krishnamurthy R, Suryanarayanan R. Influence of the active pharmaceutical ingredient concentration on the physical state of mannitol-implications in freeze-drying. Pharm Res. 2005;22:1978–85.
Tang X, Pikal MJ. Design of freeze-drying processes for pharmaceuticals: practical advice. Pharm Res. 2004;21:191–200.
Williams NA, Dean T. Vial breakage by frozen mannitol solutions: correlation with thermal characteristics and effect of stereoisomerism, additives, and vial configuration. J Parenter Sci Technol. 1991;45:94–100.
Cao W, Mao C, Chen W, Lin H, Krishnan S, Cauchon N. Differentiation and quantitative determination of surface and hydrate water in lyophilized mannitol using NIR spectroscopy. J Pharm Sci. 2006;95:2077–86.
ACKNOWLEDGMENTS AND DISCLOSURES
The project was partially supported by the William and Mildred Peters endowment fund. Use of the Advanced Photon Source was supported by U.S. Department of Energy, Office of Science, Office of Basic Energy Science, under Contract No. DE-AC02-06CH11357. We thank Dr. Wenqian Xu and Dr. Andrey Yakovenko for their help during the beamline experiments. Parts of this work were carried out in the Characterization Facility, University of Minnesota, which receives partial support from NSF through the MRSEC program.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 1507 kb)
Rights and permissions
About this article
Cite this article
Thakral, S., Koranne, S. & Suryanarayanan, R. Intra-Vial Heterogeneity in Physical Form of Mannitol in Colyophilized Binary Systems. Pharm Res 35, 214 (2018). https://doi.org/10.1007/s11095-018-2499-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-018-2499-x