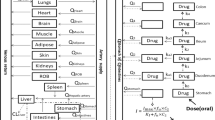
ABSTRACT
Purpose
Onset and rate of gastric emptying are important determinants of drug absorption after oral dosing. Therefore, robust estimates of these parameters are needed in physiologically based absorption models to predict reliably plasma concentration time profiles. For human and some other laboratory animals, reasonable parameterization of gastric emptying has been established. However gastric emptying is less well characterized in minipigs, a large animal model rapidly gaining importance in pharmaceutical research.
Methods
A pharmacokinetic crossover study using different dosage forms of paracetamol (intravenous and oral solution, capsule and tablet) was conducted in four male and four female Göttingen minipigs after an overnight fast. Deconvolution analysis was performed to determine the absorption kinetics. Estimated lag times and first order gastric emptying parameters were incorporated in a previously published PBPK model of the minipig and simulations verified. Postmortem assessments of minipig stomachs were made after different fasting protocols.
Results
Fraction of dose absorbed vs. time profiles showed high interindividual variability, comparable to human fed state absorption. Mean gastric transit times were determined to be 0.63 h, 1.36 h, and 0.73 h for solution, capsules, and tablets, respectively. Postmortem assessment confirmed that minipig stomachs were not empty after an overnight fast.
Conclusions
Gastric transit times in overnight fasted minipigs are longer than those observed in humans. This is most likely caused by delayed and incomplete food emptying and further work is needed to develop feasible and effective fasting protocols for minipigs.







Similar content being viewed by others
REFERENCES
Suenderhauf C, Hammann F, Maunz A, Helma C, Huwyler J. Combinatorial QSAR modeling of human intestinal absorption. Mol Pharm. 2011;8(1):213–24.
Fleisher D, Li C, Zhou Y, Pao LH, Karim A. Drug, meal and formulation interactions influencing drug absorption after oral administration. Clinical implications. Clin Pharmacokinet. 1999;36(3):233–54.
Sanjeevi A. Gastric motility. Curr Opin Gastroenterol. 2007;23(6):625–30.
Jones HM, Parrott N, Jorga K, Lave T. A novel strategy for physiologically based predictions of human pharmacokinetics. Clin Pharmacokinet. 2006;45(5):511–42.
Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos. 1995;16(5):351–80.
Forster R, Bode G, Ellegaard L, van der Laan JW. The RETHINK project on minipigs in the toxicity testing of new medicines and chemicals: conclusions and recommendations. J Pharmacol Toxicol Methods. 2010;62(3):236–42.
Monteiro-Riviere NA, Bristol DG, Manning TO, Rogers RA, Riviere JE. Interspecies and interregional analysis of the comparative histologic thickness and laser Doppler blood flow measurements at five cutaneous sites in nine species. J Investig Dermatol. 1990;95(5):582–6.
Swindle MM, editor. Swine in the laboratory: surgery, anesthesia, imaging and experimental techniques. 2nd ed. Boca Raton: CRC Press; 2007.
Bode G, Clausing P, Gervais F, Loegsted J, Luft J, Nogues V, et al. The utility of the minipig as an animal model in regulatory toxicology. J Pharmacol Toxicol Methods. 2010;62(3):196–220.
Suenderhauf C, Parrott N. A physiologically based pharmacokinetic model of the minipig: data compilation and model implementation. Pharm Res. 2013;30(1):1–15.
Köttendorf S. Auswirkungen der Vermahlungsintensität (grob, fein) und Konfektionierung (schrotförmig, pelletiert) des Mischfutters auf die Milieubedingungen im Mageninhalt von Schweinen. In: Tierärztliche Hochschule Hannover. Hannover: University of Hannover; 2009. p. 225.
Hänichen T. Stomach ulcers in swine. Tierarztl Prax. 1975;2:191–7.
Bal HS, Ghoshal NG. Histomorphology of the torus pyloricus of the domestic pig (Sus scrofa domestica). Zentralbl Veterinarmed Reihe C Anat Histol Embryol. 1972;1(4):289–98.
Hossain M, Abramowitz W, Watrous BJ, Szpunar GJ, Ayres JW. Gastrointestinal transit of nondisintegrating, nonerodible oral dosage forms in pigs. Pharm Res. 1990;7(11):1163–6.
Oberle RL, Das H. Variability in gastric pH and delayed gastric emptying in Yucatan miniature pigs. Pharm Res. 1994;11(4):592–4.
Merchant HA, McConnell EL, Liu F, Ramaswamy C, Kulkarni RP, Basit AW, et al. Assessment of gastrointestinal pH, fluid and lymphoid tissue in the guinea pig, rabbit and pig, and implications for their use in drug development. Eur J Pharm Sci Off J Eur Fed Pharm Sci. 2011;42(1–2):3–10.
DeSesso JM, Williams AL. Contrasting the gastrointestinal tracts of mammals: factors that influence absorption. In: Macor JE, editor. Annual reports in medicinal chemistry. The Netherlands: Academic; 2008. p. 353–71.
Wilfart A, Montagne L, Simmins H, Noblet J, Milgen J. Digesta transit in different segments of the gastrointestinal tract of pigs as affected by insoluble fibre supplied by wheat bran. B J Nutr. 2007;98(1):54–62.
Aoyagi N, Ogata H, Kaniwa N, Ejima A, Yasuda Y, Tanioka Y. Bioavailability of griseofulvin from plain tablets in Gottingen minipigs and the correlation with bioavailability in humans. J Pharmacobiodyn. 1984;7(1):7–14.
Naslund E, Bogefors J, Gryback P, Jacobsson H, Hellstrom PM. Gastric emptying: comparison of scintigraphic, polyethylene glycol dilution, and paracetamol tracer assessment techniques. Scand J Gastroenterol. 2000;35(4):375–9.
Benet LZ, Broccatelli F, Oprea TI. BDDCS applied to over 900 drugs. AAPS J. 2011;13(4):519–47.
Forrest JA, Clements JA, Prescott LF. Clinical pharmacokinetics of paracetamol. Clin Pharmacokinet. 1982;7(2):93–107.
Willems M, Quartero AO, Numans ME. How useful is paracetamol absorption as a marker of gastric emptying? A systematic literature study. Dig Dis Sci. 2001;46(10):2256–62.
Finnegan BA, Jyn CA. Paracetamol kinetics and gastric emptying. Br J Anaesth. 1985;57(10):1039–40.
Clements JA, Heading RC, Nimmo WS, Prescott LF. Kinetics of acetaminophen absorption and gastric emptying in man. Clin Pharmacol Ther. 1978;24(4):420–31.
Heading RC, Nimmo J, Prescott LF, Tothill P. The dependence of paracetamol absorption on the rate of gastric emptying. Br J Pharmacol. 1973;47(2):415–21.
Mogi M, Toda A, Iwasaki K, Kusumoto S, Takehara H, Shimizu M, et al. Simultaneous pharmacokinetics assessment of caffeine, warfarin, omeprazole, metoprolol, and midazolam intravenously or orally administered to Microminipigs. J Toxicol Sci. 2012;37(6):1157–64.
Agoram B, Woltosz WS, Bolger MB. Predicting the impact of physiological and biochemical processes on oral drug bioavailability. Adv Drug Deliv Rev. 2001;50 Suppl 1:S41–67.
Parrott N, Lave T. Applications of physiologically based absorption models in drug discovery and development. Mol Pharm. 2008;5(5):760–75.
Rodgers T, Leahy D, Rowland M. Tissue distribution of basic drugs: accounting for enantiomeric, compound and regional differences amongst beta-blocking drugs in rat. J Pharm Sci. 2005;94(6):1237–48.
A/S, E.G.M., Minipig background data. 2013: Dalmose.
Levitt DG. Quantitation of small intestinal permeability during normal human drug absorption. BMC Pharmacol Toxicol. 2013;14:34.
Neirinckx E, Vervaet C, Michiels J, De Smet S, Van den Broeck W, Remon JP, et al. Feasibility of the Ussing chamber technique for the determination of in vitro jejunal permeability of passively absorbed compounds in different animal species. J Vet Pharmacol Ther. 2011;34(3):290–7.
Sjogren E, Bredberg U, Lennernas H. The pharmacokinetics and hepatic disposition of repaglinide in pigs: mechanistic modeling of metabolism and transport. Mol Pharm. 2012;9(4):823–41.
Khan S, Elshaer A, Rahman AS, Hanson P, Perrie Y, Mohammed AR. Systems biology approach to study permeability of paracetamol and its solid dispersion. Int J Pharm. 2011;417(1–2):272–9.
Neirinckx E, Vervaet C, De Boever S, Remon JP, Gommeren K, Daminet S, et al. Species comparison of oral bioavailability, first-pass metabolism and pharmacokinetics of acetaminophen. Res Vet Sci. 2010;89(1):113–9.
Skaanild MT, Friis C. Cytochrome P450 sex differences in minipigs and conventional pigs. Pharmacol Toxicol. 1999;85(4):174–80.
Rawlins MD, Henderson DB, Hijab AR. Pharmacokinetics of paracetamol (acetaminophen) after intravenous and oral administration. Eur J Clin Pharmacol. 1977;11(4):283–6.
Chiou WL. Estimation of hepatic first-pass effect of acetaminophen in humans after oral administration. J Pharm Sci. 1975;64(10):1734–5.
Perucca E, Richens A. Paracetamol disposition in normal subjects and in patients treated with antiepileptic drugs. Br J Clin Pharmacol. 1979;7(2):201–6.
Baranova J, Anzenbacherova E, Anzenbacher P, Soucek P. Minipig cytochrome P450 2E1: comparison with human enzyme. Drug Metab Dispos Biol Fate Chem. 2005;33(6):862–5.
Hinson JA. Reactive metabolites of phenacetin and acetaminophen: a review. Environ Health Perspect. 1983;49:71–9.
Laine JE, Auriola S, Pasanen M, Juvonen RO. Acetaminophen bioactivation by human cytochrome P450 enzymes and animal microsomes. Xenobiotica Fate Foreign Compd Biol Syst. 2009;39(1):11–21.
Oberle RL, Das H, Wong SL, Chan KK, Sawchuk RJ. Pharmacokinetics and metabolism of diclofenac sodium in Yucatan miniature pigs. Pharm Res. 1994;11(5):698–703.
Oberle RL, Amidon GL. The influence of variable gastric emptying and intestinal transit rates on the plasma level curve of cimetidine; an explanation for the double peak phenomenon. J Pharmacokinet Biopharm. 1987;15(5):529–44.
Hayashi M, Tomita M. Mechanistic analysis for drug permeation through intestinal membrane. Drug Metab Pharmacokinet. 2007;22(2):67–77.
Yazdanian M, Glynn SL, Wright JL, Hawi A. Correlating partitioning and caco-2 cell permeability of structurally diverse small molecular weight compounds. Pharm Res. 1998;15(9):1490–4.
Watanabe K, Furuno K, Eto K, Oishi R, Gomita Y. First-pass metabolism of omeprazole in rats. J Pharm Sci. 1994;83(8):1131–4.
Andersson T, Cederberg C, Regardh CG, Skanberg I. Pharmacokinetics of various single intravenous and oral doses of omeprazole. Eur J Clin Pharmacol. 1990;39(2):195–7.
Pilbrant A, Cederberg C. Development of an oral formulation of omeprazole. Scand J Gastroenterol Suppl. 1985;108:113–20.
Kanazu T, Yamaguchi Y, Okamura N, Baba T, Koike M. Model for the drug-drug interaction responsible for CYP3A enzyme inhibition. I: evaluation of cynomolgus monkeys as surrogates for humans. Xenobiotica Fate Foreign Compd Biol Syst. 2004;34(5):391–402.
Strelevitz TJ, Foti RS, Fisher MB. In vivo use of the P450 inactivator 1-aminobenzotriazole in the rat: varied dosing route to elucidate gut and liver contributions to first-pass and systemic clearance. J Pharm Sci. 2006;95(6):1334–41.
Komura H, Iwaki M. Species differences in in vitro and in vivo small intestinal metabolism of CYP3A substrates. J Pharm Sci. 2008;97(5):1775–800.
Pauli-Magnus C, Rekersbrink S, Klotz U, Fromm M. Interaction of omeprazole, lansoprazole and pantoprazole with P-glycoprotein. Naunyn Schmiedeberg’s Arch Pharmacol. 2001;364(6):551–7.
Tolle-Sander S, Rautio J, Wring S, Polli J, Polli J. Midazolam exhibits characteristics of a highly permeable P-glycoprotein substrate. Pharm Res. 2003;20(5):757–64.
Nusynowitz ML, Benedetto AR. The lag phase of gastric emptying: clinical, mathematical and in vitro studies. J Nucl Med Off Publ Soc Nucl Med. 1994;35(6):1023–7.
Hellmig S, Von Schoning F, Gadow C, Katsoulis S, Hedderich J, Folsch UR, et al. Gastric emptying time of fluids and solids in healthy subjects determined by 13C breath tests: influence of age, sex and body mass index. J Gastroenterol Hepatol. 2006;21(12):1832–8.
Köttendorf S. Auswirkungen der Vermahlungsintensität (grob, fein) und Konfektionierung (schrotförmig, pelletiert) des Mischfutters auf die Milieubedingungen im Mageninhalt von Schweinen. Gießen: Deutsche Veterinärmedizinische Gesellschaft Service GmbH; 2009.
Madsen JL. Effects of gender, age, and body mass index on gastrointestinal transit times. Dig Dis Sci. 1992;37(10):1548–53.
Hou SY, Cowles VE, Berner B. Gastric retentive dosage forms: a review. Crit Rev Ther Drug Carrier Syst. 2003;20(6):459–97.
Johansson UB, Eskils J, Adamson U, Elwin CE, Wredling R, Lins PE. A paracetamol-pasta test for assessing gastric emptying in healthy and diabetic subjects. Scand J Clin Lab Invest. 2003;63(2):159–66.
Thomsen C, Rasmussen OW, Christiansen C, Andreasen F, Poulsen PL, Hermansen K. The glycaemic index of spaghetti and gastric emptying in non-insulin-dependent diabetic patients. Eur J Clin Nutr. 1994;48(11):776–80.
Wyse CA, Marshall WG, Preston T, Yam PS. Retention of acetaminophen in an in vitro model of solid-phase gastric emptying of animals. Am J Vet Res. 2007;68(8):895–8.
ACKNOWLEDGMENTS AND DISCLOSURES
H. Lorentsen is employed by the minipig provider Ellegaard Göttingen Minipigs A/S.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 22.4 kb)
Rights and permissions
About this article
Cite this article
Suenderhauf, C., Tuffin, G., Lorentsen, H. et al. Pharmacokinetics of Paracetamol in Göttingen Minipigs: In Vivo Studies and Modeling to Elucidate Physiological Determinants of Absorption. Pharm Res 31, 2696–2707 (2014). https://doi.org/10.1007/s11095-014-1367-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1367-6