Abstract
Purpose
To identify the effects of cross-linkers and drug-binding linkers on physicochemical and biological properties of polymer nanoassembly drug carriers.
Methods
Four types of polymer nanoassemblies were synthesized from poly(ethylene glycol)-poly(aspartate) [PEG-p(Asp)] block copolymers: self-assembled nanoassemblies (SNAs) and cross-linked nanoassemblies (CNAs) to each of which an anticancer drug doxorubicin (DOX) was loaded by either physical entrapment or chemical conjugation (through acid-sensitive hydrazone linkers).
Results
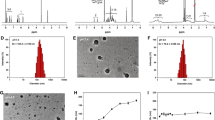
Drug loading in nanoassemblies was 27 ~ 56% by weight. The particle size of SNA changed after drug and drug-binding linker entrapment (20 ~ 100 nm), whereas CNAs remained 30 ~ 40 nm. Drug release rates were fine-tunable by using amide cross-linkers and hydrazone drug-binding linkers in combination. In vitro cytotoxicity assays using a human lung cancer A549 cell line revealed that DOX-loaded nanoassemblies were equally potent as free DOX with a wide range of drug release half-life (t1/2 = 3.24 ~ 18.48 h, at pH 5.0), but 5 times less effective when t1/2 = 44.52 h.
Conclusion
Nanoassemblies that incorporate cross-linkers and drug-binding linkers in combination have pharmaceutical advantages such as uniform particle size, physicochemical stability, fine-tunable drug release rates, and maximum cytotoxicity of entrapped drug payloads.





Similar content being viewed by others
References
Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3:16–20.
Yokoyama M. Polymeric micelles as a new drug carrier system and their required considerations for clinical trials. Exp Opin Drug Del. 2010;7:145–58.
Cabral H, Kataoka K. Multifunctional nanoassemblies of block copolymers for future cancer therapy. Sci Technol Adv Mat. 2010:11
Victor EG, Silveira PCL, Possato JC, da Rosa GL, Munari UB, de Souza CT, Pinho RA, da Silva L, Streck EL, Paula MMS. Pulsed ultrasound associated with gold nanoparticle gel reduces oxidative stress parameters and expression of pro-inflammatory molecules in an animal model of muscle injury. J Nanobiotechnol. 2012:10
Bisht S, Khan MA, Bekhit M, Bai HB, Cornish T, Mizuma M, Rudek MA, Zhao M, Maitra A, Ray B, Lahiri D, Maitra A, Anders RA. A polymeric nanoparticle formulation of curcumin (NanoCurc (TM)) ameliorates CCl4-induced hepatic injury and fibrosis through reduction of pro-inflammatory cytokines and stellate cell activation. Lab Invest. 2011;91:1383–95.
Fang J, Qin HB, Nakamura H, Tsukigawa K, Shin T, Maeda H. Carbon monoxide, generated by heme oxygenase-1, mediates the enhanced permeability and retention effect in solid tumors. Cancer Sci. 2012;103:535–41.
Sancey L, Barbier E, Hirsjarvi S, Dufort S, Benoit JP, Remy C, Coll JL. Enhanced Permeability and Retention (EPR) effect in tumors: characterization by MRI and fluorescence imaging. B Cancer. 2011;98:S67.
Santi DV, Schneider EL, Reid R, Robinson L, Ashley GW. Predictable and tunable half-life extension of therapeutic agents by controlled chemical release from macromolecular conjugates. PNAS. 2012;109:6211–6.
Wei CA, Guo J, Wang CC. Dual stimuli-responsive polymeric micelles exhibiting “AND” logic gate for controlled release of adriamycin. Macromol Rapid Comm. 2011;32:451–5.
Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63:136–51.
Miyata K, Nishiyama N, Kataoka K. Rational design of smart supramolecular assemblies for gene delivery: chemical challenges in the creation of artificial viruses. Chem Soc Rev. 2012;41:2562–74.
Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–8.
Solier S, Zhang YW, Ballestrero A, Pommier Y, Zoppoli G. DNA damage response pathways and cell cycle checkpoints in colorectal cancer: current concepts and future perspectives for targeted treatment. Curr Cancer Drug Tar. 2012;12:356–71.
Drummond DC, Noble CO, Hayes ME, Park JW, Kirpotin DB. Pharmacokinetics and in vivo drug release rates in liposomal nanocarrier development. J Pharm Sci. 2008;97:4696–740.
Pamies P. Optimized for the clinic. Nat Mater. 2012;11:358.
Siepmann J, Siepmann F. Mathematical modeling of drug delivery. Int J Pharm. 2008;364:328–43.
Croy SR, Kwon GS. Polymeric micelles for drug delivery. Curr Pharm Des. 2006;12:4669–84.
Kwon GS, Forrest ML. Amphiphilic block copolymer micelles for nanoscale drug delivery. Drug Dev Res. 2006;67:15–22.
Beheshti N, Kjoniksen AL, Zhu KZ, Knudsen KD, Nystrom B. Viscosification in polymer-surfactant mixtures at low temperatures. J Phys Chem B. 2010;114:6273–80.
Lu CH, Mikhail AS, Wang XY, Brook MA, Allen C. Hydrogels containing core cross-linked block co-polymer micelles. J Biomat Sci-Polym E. 2012;23:1069–90.
Oberoi HS, Nukolova NV, Laquer FC, Poluektova LY, Huang JG, Alnouti Y, Yokohira M, Arnold LL, Kabanov AV, Cohen SM, Bronich TK. Cisplatin-loaded core cross-linked micelles: comparative pharmacokinetics, antitumor activity, and toxicity in mice. Int J Nanomed. 2012;7:2557–71.
Gillies ER, Frechet JMJ. Development of acid-sensitive copolymer micelles for drug delivery. Pure Appl Chem. 2004;76:1295–307.
Sato T, Matsuda Y. Macromolecular assemblies in solution: characterization by light scattering. Polym J. 2009;41:241–51.
Tachibana Y, Nakazono K, Takata T. Self-assembly of macromolecular threaded systems. Supramol Chem. 2012;5:2207–23.
Elsabahy M, Wooley KL. Strategies toward well-defined polymer nanoparticles inspired by nature: chemistry versus versatility. J Polym Sci Pol Chem. 2012;50:1869–80.
van Nostrum CF. Covalently cross-linked amphiphilic block copolymer micelles. Soft Matter. 2011;7:3246–59.
Kim JO, Sahay G, Kabanov AV, Bronich TK. Polymeric micelles with ionic cores containing biodegradable cross-links for delivery of chemotherapeutic agents. Biomacromolecules. 2010;11:919–26.
Bae Y, Kataoka K. Intelligent polymeric micelles from functional poly(ethylene glycol)-poly(amino acid) block copolymers. Adv Drug Deliv Rev. 2009;61:768–84.
Lee HJ, Ponta A, Bae Y. Polymer nanoassemblies for cancer treatment and imaging. Ther Deliv. 2010;1:803–17.
Ponta A, Bae Y. PEG-poly(amino acid) block copolymer micelles for tunable drug release. Pharm Res. 2010;27:2330–42.
Eckman AM, Tsakalozou E, Kang NY, Ponta A, Bae Y. Drug release patterns and cytotoxicity of PEG-poly(aspartate) block copolymer micelles in cancer cells. Pharm Res. 2012
Alani AWG, Bae Y, Kwon GS. Synthesis and characterization of a micellar drug carrier for the metronomic delivery of paclitaxel. J Control Release. 2008;132:e19–20.
Scott D, Rohr J, Bae Y. Nanoparticulate formulations of mithramycin analogs for enhanced cytotoxicity. Int J Nanomed. 2011;6:2757–67.
Le Tourneau C, Dieras V, Tresca P, Cacheux W, Paoletti X. Current challenges for the early clinical development of anticancer drugs in the era of molecularly targeted agents. Target Oncol. 2010;5:65–72.
Atkins JH, Gershell LJ. Selective anticancer drugs. Nat Rev Drug Discov. 2002;1:491–2.
Xu L, Anchordoquy T. Drug delivery trends in clinical trials and translational medicine: challenges and opportunities in the delivery of nucleic acid-based therapeutics. J Pharm Sci. 2011;100:38–52.
Ruenraroengsak P, Cook JM, Florence AT. Nanosystem drug targeting: facing up to complex realities. J Control Release. 2010;141:265–76.
Sanhai WR, Sakamoto JH, Canady R, Ferrari M. Seven challenges for nanomedicine. Nat Nanotechnol. 2008;3:242–4.
Grattoni A, Shen H, Fine D, Ziemys A, Gill JS, Hudson L, Hosali S, Goodall R, Liu X, Ferrari M. Nanochannel technology for constant delivery of chemotherapeutics: beyond metronomic administration. Pharm Res. 2011;28:292–300.
Gorn M, Habermann CR, Anige M, Thom I, Schuch G, Andritzky B, Brandl S, Burkholder I, Edler L, Hossfeld DK, Bokemeyer C, Laack E. A pilot study of docetaxel and trofosfamide as second-line ‘metronomic’ chemotherapy in the treatment of metastatic non-small cell lung cancer (NSCLC). Onkologie. 2008;31:185–9.
Jain A, Jain SK. PEGylation: an approach for drug delivery. A review. Crit Rev Ther Drug Carrier Syst. 2008;25:403–47.
Nel AE, Madler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V, Thompson M. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8:543–57.
Vasir JK, Labhasetwar V. Quantification of the force of nanoparticle-cell membrane interactions and its influence on intracellular trafficking of nanoparticles. Biomaterials. 2008;29:4244–52.
Lee HJ, Bae Y. Cross-linked nanoassemblies from poly(ethylene glycol)-poly(aspartate) block copolymers as stable supramolecular templates for particulate drug delivery. Biomacromolecules. 2011;12:2686–96.
Bae Y, Alani AWG, Rockich NC, Lai TSZC, Kwon GS. Mixed pH-sensitive polymeric micelles for combination drug delivery. Pharm Res. 2010;27:2421–32.
Alani AWG, Bae Y, Rao DA, Kwon GS. Polymeric micelles for the pH-dependent controlled, continuous low dose release of paclitaxel. Biomaterials. 2010;31:1765–72.
Kano MR, Bae Y, Iwata C, Morishita Y, Yashiro M, Oka M, Fujii T, Komuro A, Kiyono K, Kaminishi M, Hirakawa K, Ouchi Y, Nishiyama N, Kataoka K, Miyazono K. Improvement of cancer-targeting therapy, using nanocarriers for intractable solid tumors by inhibition of TGF-β signaling. PNAS. 2007;104:3460–5.
Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5:505–15.
Zhang K, Fang H, Chen Z, Taylor John-Stephen A, Wooley Karen L. Shape effects of nanoparticles conjugated with cell-penetrating peptides (HIV Tat PTD) on CHO cell uptake. Bioconjugate Chem. 2008;19:1880–7.
Decuzzi P, Gentile F, Granaldi A, Curcio A, Causa F, Indolfi C, Netti P, Ferrari M. Flow chamber analysis of size effects in the adhesion of spherical particles. Int J Nanomedicine. 2007;2:689–96.
Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, Terada Y, Kano MR, Miyazono K, Uesaka M, Nishiyama N, Kataoka K. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat Nanotechnol. 2011;6:815–23.
Anraku Y, Kishimura A, Kobayashi A, Oba M, Kataoka K. Size-controlled long-circulating PICsome as a ruler to measure critical cut-off disposition size into normal and tumor tissues. Chem Commun. 2011;47:6054–6.
Untch M, Loibl S, Konecny GE, von Minckwitz G. Neoadjuvant clinical trials for the treatment of primary breast cancer: the experience of the german study groups. Curr Oncol Rep. 2012;14:27–34.
Falchook GS, Duvic M, Hong DS, Wheler J, Naing A, Lim J, Kurzrock R. Age-stratified phase I trial of a combination of bortezomib, gemcitabine, and liposomal doxorubicin in patients with advanced malignancies. Cancer Chemother Pharmacol. 2012;69:1117–26.
Bae Y, Nishiyama N, Kataoka K. In Vivo antitumor activity of the folate-conjugated pH-sensitive polymeric micelle selectively releasing adriamycin in the intracellular acidic compartments. Bioconjugate Chem. 2007;18:1131–9.
Yokoyama M, Inoue S, Kataoka K, Yui N, Sakurai Y. Preparation of adriamycin-conjugated poly(ethylene glycol)-poly(aspartic acid) block copolymer - a new type of polymeric anticancer agent. Makromol Chem-Rapid. 1987;8:431–5.
Bae Y, Jang W-D, Nishiyama N, Fukushima S, Kataoka K. Multifunctional polymeric micelles with folate-mediated cancer cell targeting and pH-triggered drug releasing properties for active intracellular drug delivery. Mol Biosyst. 2005;1:242–50.
Duncan R. Designing polymer conjugates as lysosomotropic nanomedicines. Biochem Soc Trans. 2007;35:56–60.
Matsumura Y, Kataoka K. Preclinical and clinical studies of anticancer agent-incorporating polymer micelles. Cancer Sci. 2009;100:572–9.
Vicent MJ, Ringsdorf H, Duncan R. Polymer therapeutics: clinical applications and challenges for development. Adv Drug Deliv Rev. 2009;61:1117–20.
Peppas NA. Drug delivery using smart polymers: recent advances. Smart Polym. 2008:331–358
Satchi-Fainaro R, Duncan R, Barnes CM. Polymer therapeutics for cancer: current status and future challenges. Adv Polym Sci. 2006;193:1–65.
Torchilin VP. Nanocarriers. Pharm Res. 2007;24:2333–4.
Bae Y, Nishiyama N, Fukushima S, Koyama H, Yasuhiro M, Kataoka K. Preparation and biological characterization of polymeric micelle drug carriers with intracellular pH-triggered drug release property: tumor permeability, controlled subcellular drug distribution, and enhanced in vivo antitumor efficacy. Bioconjugate Chem. 2005;16:122–30.
Caldorera-Moore M, Guimard N, Shi L, Roy K. Designer nanoparticles: incorporating size, shape and triggered release into nanoscale drug carriers. Expert Opin Drug Deliv. 2010;7:479–95.
Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9:615–27.
Dobrovolskaia MA, Germolec DR, Weaver JL. Evaluation of nanoparticle immunotoxicity. Nat Nanotechnol. 2009;4:411–4.
AcknowledgmentS AND DISCLOSURES
This research is supported by the Kentucky Lung Cancer Research Program. Authors thank Alli Eckman for assisting cytotoxicity assays.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, H.J., Bae, Y. Pharmaceutical Differences Between Block Copolymer Self-Assembled and Cross-Linked Nanoassemblies as Carriers for Tunable Drug Release. Pharm Res 30, 478–488 (2013). https://doi.org/10.1007/s11095-012-0893-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-012-0893-3