ABSTRACT
Purpose
Camptothecin analogues are anticancer drugs effective when dosed in protracted schedules. Such treatment is best suited for oral formulations. AR-67 is a novel lipophilic analogue with potent efficacy in preclinical models. Here we assessed factors that may influence its oral bioavailability in rats.
Methods
Plasma pharmacokinetic (PK) studies were conducted following administration of AR-67 lactone or carboxylate doses alone or after pre-dosing with inhibitors of the efflux transporters P-gp and Bcrp. A population PK model that simultaneously fitted to oral and intravenous data was used to estimate the bioavailability (F) and clearance of AR-67.
Results
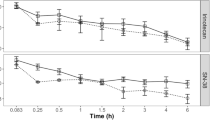
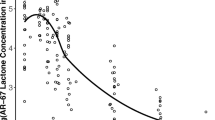
An inverse Gaussian function was used as the oral input into the model and provided the best fits. Covariate analysis showed that the bioavailability of the lactone, but not its clearance, was dose dependent. Consistent with this observation, the bioavailability of AR-67 increased when animals were pretreated orally with GF120918 or Zosuquidar.
Conclusion
Absorption of AR-67 is likely affected by solubility of its lactone form and interaction with efflux pumps in the gut. AR-67 appears to be absorbed as the lactone form, most likely due to gastric pH favoring its formation and predominance. F increased at higher doses suggesting saturation of efflux mechanisms.









Similar content being viewed by others
Abbreviations
- AUC:
-
area under the plasma concentration versus time curve
- BCRP/bcrp:
-
breast cancer resistance protein
- OATP/Oatp:
-
organic anion transporting polypeptide
- P-gp:
-
p-glycoprotein
REFERENCES
Bom D, Curran DP, Zhang J, Zimmer SG, Bevins R, Kruszewski S, et al. The highly lipophilic DNA topoisomerase I inhibitor DB-67 displays elevated lactone levels in human blood and potent anticancer activity. J Contr Release. 2001;74:325–33.
Arnold SM, Rinehart JJ, Tsakalozou E, Eckardt JR, Fields SZ, Shelton BJ, et al. A phase I study of 7-t-butyldimethylsilyl-10-hydroxycamptothecin in adult patients with refractory or metastatic solid malignancies. Clin Cancer Res. 2010;16:673–80.
Zhu AX, Ready N, Clark JW, Safran H, Amato A, Salem N, et al. Phase I and pharmacokinetic study of gimatecan given orally once a week for 3 of 4 weeks in patients with advanced solid tumors. Clin Cancer Res. 2009;15:374–81.
Nam EJ, Kim JW, Kim JH, Kim S, Kim SW, Jang SY, Lee DW, Jung YW, Kim YT. Efficacy and toxicity of belotecan with and without cisplatin in patients with recurrent ovarian cancer. Am J Clin Oncol. 2009.
Furman WL, Stewart CF, Poquette CA, Pratt CB, Santana VM, Zamboni WC, et al. Direct translation of a protracted irinotecan schedule from a xenograft model to a phase I trial in children. J Clin Oncol. 1999;17:1815–24.
Houghton PJ, Cheshire PJ, Hallman 2nd JD, Lutz L, Friedman HS, Danks MK, et al. Efficacy of topoisomerase I inhibitors, topotecan and irinotecan, administered at low dose levels in protracted schedules to mice bearing xenografts of human tumors. Canc Chemother Pharmacol. 1995;36:393–403.
Santana VM, Zamboni WC, Kirstein MN, Tan M, Liu T, Gajjar A, et al. A pilot study of protracted topotecan dosing using a pharmacokinetically guided dosing approach in children with solid tumors. Clin Cancer Res. 2003;9:633–40.
Liu G, Franssen E, Fitch MI, Warner E. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol. 1997;15:110–5.
Ramlau R, Gervais R, Krzakowski M, von Pawel J, Kaukel E, Abratt RP, et al. Phase III study comparing oral topotecan to intravenous docetaxel in patients with pretreated advanced non-small-cell lung cancer. J Clin Oncol. 2006;24:2800–7.
Clarke-Pearson DL, Van Le L, Iveson T, Whitney CW, Hanjani P, Kristensen G, et al. Oral topotecan as single-agent second-line chemotherapy in patients with advanced ovarian cancer. J Clin Oncol. 2001;19:3967–75.
Drengler RL, Kuhn JG, Schaaf LJ, Rodriguez GI, Villalona-Calero MA, Hammond LA, et al. Phase I and pharmacokinetic trial of oral irinotecan administered daily for 5 days every 3 weeks in patients with solid tumors. J Clin Oncol. 1999;17:685–96.
Dumez H, Awada A, Piccart M, Assadourian S, Semiond D, Guetens G, et al. A phase I dose-finding clinical pharmacokinetic study of an oral formulation of irinotecan (CPT-11) administered for 5 days every 3 weeks in patients with advanced solid tumours. Ann Oncol. 2006;17:1158–65.
Soepenberg O, Dumez H, Verweij J, de Jong FA, de Jonge MJ, Thomas J, et al. Phase I pharmacokinetic, food effect, and pharmacogenetic study of oral irinotecan given as semisolid matrix capsules in patients with solid tumors. Clin Cancer Res. 2005;11:1504–11.
Fassberg J, Stella VJ. A kinetic and mechanistic study of the hydrolysis of camptothecin and some analogues. J Pharm Sci. 1992;81:676–84.
Xiang TX, Anderson BD. Stable supersaturated aqueous solutions of silatecan 7-t-butyldimethylsilyl-10-hydroxycamptothecin via chemical conversion in the presence of a chemically modified beta-cyclodextrin. Pharm Res. 2002;19:1215–22.
Jonker JW, Smit JW, Brinkhuis RF, Maliepaard M, Beijnen JH, Schellens JH, et al. Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. J Natl Canc Inst. 2000;92:1651–6.
Schinkel AH, Mayer U, Wagenaar E, Mol CA, van Deemter L, Smit JJ, et al. Normal viability and altered pharmacokinetics in mice lacking mdr1-type (drug-transporting) P-glycoproteins. Proc Natl Acad Sci U S A. 1997;94:4028–33.
Leggas M, Panetta JC, Zhuang Y, Schuetz JD, Johnston B, Bai F, et al. Gefitinib modulates the function of multiple ATP-binding cassette transporters in vivo. Cancer Res. 2006;66:4802–7.
Milewska M, Horn J, Monks N, Moscow JA, Arnold SM, Leggas M. Metabolism and transport pathways of the blood stable camptothecin AR-67 (7-t-butyldimethylsilyl-10-hydroxycamptothecin). J Clin Oncol. 2009;27:(Abstract 2553).
Xiang T, Anderson BD. Influence of a transmembrane protein on the permeability of small molecules across lipid membranes. J Membr Biol. 2000;173:187–201.
Tong W-Q, Wells ML. Parenteral pharmaceutical compositions containing GF120918A. Glaxo Wellcome INC; WO/1996/011007, 1996.
Kaddoumi A, Choi SU, Kinman L, Whittington D, Tsai CC, Ho RJ, et al. Inhibition of P-glycoprotein activity at the primate blood-brain barrier increases the distribution of nelfinavir into the brain but not into the cerebrospinal fluid. Drug Metabol Dispos. 2007;35:1459–62.
Anderson BD, May MJ, Jordan S, Song L, Roberts MJ, Leggas M. Dependence of nelfinavir brain uptake on dose and tissue concentrations of the selective P-glycoprotein inhibitor zosuquidar in rats. Drug Metabol Dispos. 2006;34:653–9.
Yang J, Jamei M, Yeo KR, Rostami-Hodjegan A, Tucker GT. Misuse of the well-stirred model of hepatic drug clearance. Drug Metabol Dispos. 2007;35:501–2.
Adane ED, Liu Z, Xiang TX, Anderson BD, Leggas M. Factors affecting the in vivo lactone stability and systemic clearance of the lipophilic camptothecin analogue AR-67. Pharm Res. 2010;27:1416–25.
Daviesand B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10:1093–5.
Horn J, Jordan SL, Song L, Roberts MJ, Anderson BD, Leggas M. Validation of an HPLC method for analysis of DB-67 and its water soluble prodrug in mouse plasma. J Chromatogr B Anal Tech Biomed Life Sci. 2006;844:15–22.
D'Argenio DZ, Schumitzky A, Wang X. ADAPT 5 user’s guide: pharmacokinetic/pharmacodynamic systems analysis software. Los Angeles: Biomedical Simulations Resource; 2009.
Weiss M. A novel extravascular input function for the assessment of drug absorption in bioavailability studies. Pharm Res. 1996;13:1547–53.
Wang J, Weiss M, D'Argenio DZ. A note on population analysis of dissolution-absorption models using the inverse Gaussian function. J Clin Pharmacol. 2008;48:719–25.
Dantzig AH, Shepard RL, Cao J, Law KL, Ehlhardt WJ, Baughman TM, et al. Reversal of P-glycoprotein-mediated multidrug resistance by a potent cyclopropyldibenzosuberane modulator, LY335979. Cancer Res. 1996;56:4171–9.
Allen JD, Brinkhuis RF, Wijnholds J, Schinkel AH. The mouse Bcrp1/Mxr/Abcp gene: amplification and overexpression in cell lines selected for resistance to topotecan, mitoxantrone, or doxorubicin. Cancer Res. 1999;59:4237–41.
Maliepaard M, van Gastelen MA, Tohgo A, Hausheer FH, van Waardenburg RCAM, de Jong LA, et al. Circumvention of Breast Cancer Resistance Protein (BCRP)-mediated resistance to camptothecins in vitro using non-substrate drugs or the BCRP inhibitor GF120918. Clin Cancer Res. 2001;7:935–41.
Bom D, Curran DP, Kruszewski S, Zimmer SG, Thompson Strode J, Kohlhagen G, et al. The novel silatecan 7-tert-butyldimethylsilyl-10-hydroxycamptothecin displays high lipophilicity, improved human blood stability, and potent anticancer activity. J Med Chem. 2000;43:3970–80.
Scott DO, Bindra DS, Sutton SC, Stella VJ. Urinary and biliary disposition of the lactone and carboxylate forms of 20(S)-camptothecin in rats. Drug Metabol Dispos. 1994;22:438–42.
McConnell EL, Basit AW, Murdan S. Measurements of rat and mouse gastrointestinal pH, fluid and lymphoid tissue, and implications for in-vivo experiments. J Pharm Pharmacol. 2008;60:63–70.
Carrier RL, Miller LA, Ahmed I. The utility of cyclodextrins for enhancing oral bioavailability. J Contr Release. 2007;123:78–99.
Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413–20.
MacLean C, Moenning U, Reichel A, Fricker G. Closing the gaps: a full scan of the intestinal expression of p-glycoprotein, breast cancer resistance protein, and multidrug resistance-associated protein 2 in male and female rats. Drug Metabol Dispos. 2008;36:1249–54.
Wuand CY, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22:11–23.
Kruijtzer CM, Beijnen JH, Rosing H, ten Bokkel Huinink WW, Schot M, Jewell RC, et al. Increased oral bioavailability of topotecan in combination with the breast cancer resistance protein and P-glycoprotein inhibitor GF120918. J Clin Oncol. 2002;20:2943–50.
Stewart CF, Leggas M, Schuetz JD, Panetta JC, Cheshire PJ, Peterson J, et al. Gefitinib enhances the antitumor activity and oral bioavailability of irinotecan in mice. Cancer Res. 2004;64:7491–9.
Rowland A, Elliot DJ, Knights KM, Mackenzie PI, Miners JO. The “albumin effect” and in vitro-in vivo extrapolation: sequestration of long-chain unsaturated fatty acids enhances phenytoin hydroxylation by human liver microsomal and recombinant cytochrome P450 2C9. Drug Metabol Dispos. 2008;36:870–7.
Lee W, Belkhiri A, Lockhart AC, Merchant N, Glaeser H, Harris EI, et al. Overexpression of OATP1B3 confers apoptotic resistance in colon cancer. Cancer Res. 2008;68:10315–23.
Monks NR, Liu S, Xu Y, Yu H, Bendelow AS, Moscow JA. Potent cytotoxicity of the phosphatase inhibitor microcystin LR and microcystin analogues in OATP1B1- and OATP1B3-expressing HeLa cells. Mol Cancer Ther. 2007;6:587–98.
Yamagata T, Kusuhara H, Morishita M, Takayama K, Benameur H, Sugiyama Y. Improvement of the oral drug absorption of topotecan through the inhibition of intestinal xenobiotic efflux transporter, breast cancer resistance protein, by excipients. Drug Metabol Dispos. 2007;35:1142–8.
Zamboni WC, Bowman LC, Tan M, Santana VM, Houghton PJ, Meyer WH, et al. Interpatient variability in bioavailability of the intravenous formulation of topotecan given orally to children with recurrent solid tumors. Canc Chemother Pharmacol. 1999;43:454–60.
De Cesare M, Pratesi G, Veneroni S, Bergottini R, Zunino F. Efficacy of the novel camptothecin gimatecan against orthotopic and metastatic human tumor xenograft models. Clin Cancer Res. 2004;10:7357–64.
Acknowledgments & Disclosures
This work was supported in part by the National Institutes of Health (CA123867) and research grants from Arno Therapeutics.
Markos Leggas and Bradley D. Anderson have received research funding from Arno Therapeutics. The authors disclose no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adane, E.D., Liu, Z., Xiang, TX. et al. Pharmacokinetic Modeling to Assess Factors Affecting the Oral Bioavailability of the Lactone and Carboxylate Forms of the Lipophilic Camptothecin Analogue AR-67 in Rats. Pharm Res 29, 1722–1736 (2012). https://doi.org/10.1007/s11095-011-0617-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-011-0617-0