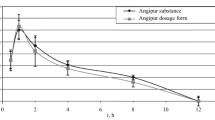
The bioavailability of the tablet dosage form of pharmaceutical substance RU-891, i.e., 9-(3,4-dihydroxyphenacyl)-2,3-dihydroimidazo[1,2-a]benzimidazole hydrobromide, was investigated. The main pharmacokinetic parameters were determined. The relative bioavailability index was estimated at 93.98%. No evidence of drug accumulation from an RU-891 270-mg tablet dosage form was detected in rabbits upon threefold administration at a dose of 23 mg/kg.


Similar content being viewed by others
References
Guideline on Bioanalytical Method Validation (EMEA 2012); http://www.ema.europa.eu.
A. A. Spasov, V. A. Anisimova, et al., RU Pat. No. 2,453,312 C1, Jun. 20, 2012; Byull., No. 17 (2010).
RF National Standard GOST R 33044-2014, Good Laboratory Practice Rules.”
Ministry of Health of Russia Order No. 199n of Apr. 1, 2016, On Approval of Good Laboratory Practice Rules.
A. N. Mironov (ed.), Handbook for Preclinical Drug Studies [in Russian], Part 1, Grif i K, Moscow (2012), pp. 80 – 93.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 56, No. 8, pp. 8 – 10, August, 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Spasov, A.A., Kucheryavenko, A.F., Gaidukova, K.A. et al. Bioavailability of the Tablet Dosage Form of a Benzimidazole Derivative Possessing Antiplatelet Activity. Pharm Chem J 56, 1024–1026 (2022). https://doi.org/10.1007/s11094-022-02746-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-022-02746-4