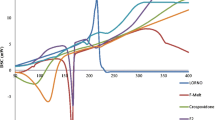
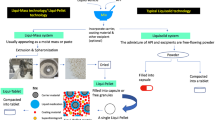
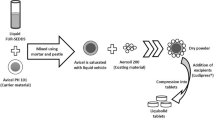
The chemical properties of ramipril and lercanidipine drug substances were studied to select the conditions necessary for technology development of solid dosage forms. Mixtures containing two or three ingredients were formulated to study the chemical compatibility of the substances and their compatibility with the excipients and were placed into storage. Analyses of these mixtures showed that the substances could be compatible in a single dosage form. Samples of intermediates (mixtures, granulates, tableting masses, tablet cores) and finished products (film-coated tablets) were manufactured, placed into storage, and analyzed to find the optimum processing factors. It was proven that the tableting process and the coexistence of ramipril and lercanidipine in any kinds of intermediates had significant effects on the ingrowth of impurities. The need to use bilayer tablet technology was justified.


Similar content being viewed by others
References
N. A. Vaulin, Consil. Medicum, No. 5, 74 – 80 (2011).
Yu. Kuligina, Farm. Vestn., No. 10, 31 (2010).
Tripartite Harmonized ICH Guideline Q8, Vialek Knowledge Book, (2011), pp. 10 – 12.
V. Bolugoddu, S. R. Mamilla, et al., US Pat. Appl. No. 2007 / 0232680, IPC A61K31 / 403, C07D209 / 02, “Preparation of ramipril and stable pharmaceutical compositions,” Oct. 4, 2007.
N. B. Dharmadhikari and V. V. Dhavse, US Pat. Appl. No. 2006 / 0045911, IPC A61K9 / 20, A61K31 / 403, “Stable pharmaceutical formulations,” Mar. 2, 2006.
N. Parmara, S. Amina, N. Singlab, and K. Kohlia, Pharm. Dev. Technol., 17, 730 – 740 (2012).
J. Fiori, R. Gotti, C. Bertucci, and V. Cavrini, J. Pharm. Biomed. Anal., 41(1), 176 – 181 (2006).
Guideline for Drug Review [in Russian], Vol. II, Grif i K, Moscow (2013).
Proceedings of the IIIrd All-Russian Scientific-Practical Conference with International Participation “Innovation in National Health” [in Russian], Nov. 10 – 11, 2015, Izd. SPKhFA, St. Petersburg (2015), pp. 490 – 492.
B. L. Moldaver, R. A. Sherstyuk, A. B. Serdyukova, and A. S. Pivovarova, Drug Incompatibilities [in Russian], SPKhFA, St. Petersburg (2003), p. 45.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 51, No. 12, pp. 24 – 29, December, 2017.
Rights and permissions
About this article
Cite this article
Shadrin, A.A., Flisyuk, E.V. & Kirillova, E.N. Compatibility of Ramipril and Lercanidipine Drug Substances in a Single Solid Dosage Form. Pharm Chem J 51, 1096–1101 (2018). https://doi.org/10.1007/s11094-018-1747-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-018-1747-z