Abstract
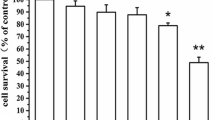
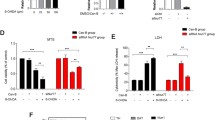
Recently, it was reported that in a 4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model, neuronal cell death is associated with the cdk5-mediated hyperphosphorylation of myocyte enhancer factor 2 (MEF2), a transcription factor that is critically required for neuronal survival. In the present study, we investigated the possible involvement of cdk5-mediated MEF2D down-regulation on 6-hydroxydopamine (6-OHDA)-induced PC12 cell death. 6-OHDA was found to significantly increase nitric oxide (NO) production and to induce apoptosis in a time-dependent manner in PC12 cells. Furthermore, 6-OHDA was found to markedly reduce MEF2D levels under conditions that could induce PC12 cell apoptosis. In addition, PC12 cell death and MEF2D degradation by 6-OHDA were prevented by the cdk5 inhibitor roscovitine, but roscovitine could not restore the 6-OHDA-induced inactivation of Akt. These results suggest that the cell death and MEF2D degradation caused by 6-OHDA are dependent on cdk5 activity. On the other hand, roscovitine enhanced the 6-OHDA-induced activations of ERK1/2 and JNK, but reduced the 6-OHDA-induced activation of p38. These results suggest that PC12 cell death by 6-OHDA appears to be regulated by the down-regulation of MEF2D via some interaction between cdk5 and MAP kinase.






Similar content being viewed by others
References
Lyons GE, Micales BK, Schwarz J et al (1995) Expression of mef2 genes in the mouse central nervous system suggests a role in neuronal maturation. J Neurosci 15:5727–5738
McKinsey TA, Zhang CL, Olson EN (2002) MEF2: a calcium-dependent regulator of cell division, differentiation and death. Trends Biochem Sci 27:40–47
Shalizi AK, Bonni A (2005) Brawn for brains: the role of MEF2 proteins in the developing nervous system. Curr Top Dev Biol 69:239–266
Black BL, Olson EN (1998) Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu Rev Cell Dev Biol 14:167–196
Mao Z, Bonni A, Xia F et al (1999) Neuronal activity-dependent cell survival mediated by transcription factor MEF2. Science 286:785–790
Mao Z, Wiedmann M (1999) Calcineurin enhances MEF2 DNA binding activity in calcium-dependent survival of cerebellar granule neurons. J Biol Chem 274:31102–31107
Okamoto S, Krainc D, Sherman K et al (2000) Antiapoptotic role of the p38 mitogen-activated protein kinase-myocyte enhancer factor 2 transcription factor pathway during neuronal differentiation. Proc Natl Acad Sci USA 97:7561–7566
Li M, Linseman DA, Allen MP et al (2001) Myocyte enhancer factor 2A and 2D undergo phosphorylation and caspase-mediated degradation during apoptosis of rat cerebellar granule neurons. J Neurosci 21:6544–6552
Gong X, Tang X, Wiedmann M et al (2003) Cdk5-mediated inhibition of the protective effects of transcription factor MEF2 in neurotoxicity-induced apoptosis. Neuron 38:33–46
Liu L, Cavanaugh JE, Wang Y et al (2003) ERK5 activation of MEF2-mediated gene expression plays a critical role in BDNF-promoted survival of developing but not mature cortical neurons. Proc Natl Acad Sci USA 100:8532–8537
Gaudilliere B, Shi Y, Bonni A (2002) RNA interference reveals a requirement for myocyte enhancer factor 2A in activity-dependent neuronal survival. J Biol Chem 277:46442–46446
Linseman DA, Bartley CM, Le SS et al (2003) Inactivation of the myocyte enhancer factor-2 repressor histone deacetylase-5 by endogenous Ca (2 +)/calmodulin-dependent kinase II promotes depolarization-mediated cerebellar granule neuron survival. J Biol Chem 278:41472–41481
Flavell SW, Cowan CW, Kim TK et al (2006) Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science 311:1008–1012
Shalizi A, Gaudillière B, Yuan Z et al (2006) A calcium-regulated MEF2 sumoylation switch controls postsynaptic differentiation. Science 311:1012–1017
Shalizi A, Bilimoria PM, Stegmüller J et al (2007) PIASx is a MEF2 SUMO E3 ligase that promotes postsynaptic dendritic morphogenesis. J Neurosci 27:10037–10046
Tang X, Wang X, Gong X et al (2005) Cyclin-Dependent Kinase 5 Mediates Neurotoxin-Induced Degradation of the Transcription Factor Myocyte Enhancer Factor 2. J Neurosci 25:4823–4834
Smith PD, Mount MP, Shree R et al (2006) Calpain-regulated p35/cdk5 plays a central role in dopaminergic neuron death through modulation of the transcription factor myocyte enhancer factor 2. J Neurosci 26:440–447
Saito Y, Nishio K, Ogawa Y et al (2007) Molecular mechanisms of 6-hydroxydopamine-induced cytotoxicity in PC12 cells: involvement of hydrogen peroxide-dependent and -independent action. Free Radic Biol Med 42:675–685
Nicholson DW, Ali A, Thornberry NA et al (1995) Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 376:37–43
Singh S, Kumar S, Dikshit M (2010) Involvement of the mitochondrial apoptotic pathway and nitric oxide synthase in dopaminergic neuronal death induced by 6-hydroxydopamine and lipopolysaccharide. Redox Rep 15:115–122
Cohen G, Heikkila RE (1974) The generation of hydrogen peroxide, superoxide radical, and hydroxyl radical by 6-hydroxydopamine, dialuric acid, and related cytotoxic agents. J Biol Chem 249:2447–2452
Liu X, Shibata T, Hisaka S, Osawa T (2009) Astaxanthin inhibits reactive oxygen species-mediated cellular toxicity in dopaminergic SH-SY5Y cells via mitochondria-targeted protective mechanism. Brain Res 1254:18–27
Bové J, Prou D, Perier C et al (2005) Toxin-induced models of Parkinson’s disease. NeuroRx 2:484–494
Blum D, Torch S, Lambeng N et al (2001) Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson’s disease. Prog Neurobiol 65:135–172
Gerlach M, Riederer P (1996) Animal models of Parkinson’s disease: an empirical comparison with the phenomenology of the disease in man. J neural Trans 103:987–1041
Sauer H, Oertel WH (1994) Progressive degeneration of nigrostriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience 59:401–415
Heikkila R, Cohen G (1971) Inhibition of biogenic amine uptake by hydrogen peroxide: a mechanism for toxic effects of 6-hydroxydopamine. Science 172:1257–1258
Permual AS, Tordzro WK, Katz M et al (1989) Regional effects of 6-hydroxydopamine on free radical scavengers in the rat brain. Brain Res 504:139–141
Permual AS, Gopal VB, Tordzro WK et al (1992) Vitamin E attenuates the toxic effects of 6-hydroxydopamine on free radical scavenging systems in rat brain. Brain Res Bull 29:699–701
Kumar R, Agarwal ML, Seth PK (1995) Free radical-generated neurotoxicity of 6-hydroxydopamine. J Neurochem 64:1703–1707
Tiffany-Castiglioni E, Saneto RP, Proctor PH et al (1982) Participation of active oxygen species in 6-hydroxydopamine toxicity to a human neuroblastoma cell line. Biochem Pharmacol 31:181–188
Decker DE, Althaus JS, Buxser SE et al (1993) Competitive irreversible inhibition of dopamine uptake by 6-hydroxydopamine. Res Commun Chem Pathol Pharmacol 79:195–208
Abad F, Maroto R, Lopez MG et al (1995) Pharmacological protection against the cytotoxicity of 6-hydroxydopamine and H2O2 in chromaffin cells. Eur J Pharmacol 293:55–64
Choi WS, Yoon SY, Oh TH et al (1999) Two distinct mechanisms are involved in 6-hydroxydopamine- and MPP+-induced dopaminergic cell death: role of caspases, ROS and JNK. J Neurosci Res 57:86–94
Lotharius J, Dugan LL, O’Malley KL (1999) Distinct mechanisms underlie neurotoxin-mediated cell death in cultured dopaminergic neurons. J Neurosci 19:1284–1293
Sharma P, Veeranna A, Sharma P, Sharma M et al (2002) Phosphorylation of MEK1 by cdk5/p35 down-regulates the mitogen-activated protein kinase pathway. J Biol Chem 277:528–534
Harada T, Morooka T, Ogawa S et al (2001) ERK induces p35, a neuron-specific activator of Cdk5, through induction of Egr1. Nat Cell Biol 3:453–459
Takai N, Nakanishi H, Tanabe K et al (1998) Involvement of caspase-like proteinases in apoptosis of neuronal PC12 cells and primary cultured microglia induced by 6-hydroxydopamine. J Neurosci Res 54:214–222
Zheng YL, Li BS, Kanungo J et al (2006) Cdk5 modulation of mitogen-activated protein kinase signaling regulates neuronal survival. Mol Biol Cell 18:404–413
Li BS, Zhang L, Takahashi S et al (2002) Cyclin-dependent kinase 5 prevents neuronal apoptosis by negative regulation of c-Jun N-terminal kinase 3. EMBO J 21:324–333
Nikolic M, Chou MM, Lu W et al (1998) The p35/Cdk5 kinase is a neuron-specific Rac effector that inhibits Pak1 activity. Nature 395:194–198
Shetty KT, Veeranna A, Takahashi M et al (2000) Cdk5 and MAPK are associated with complexes of cytoskeletal proteins in rat brain. Brain Res Mol Brain Res 76:229–236
Brunet A, Datta SR, Greenberg ME (2001) Transcription-dependent and -independent control of neuronal survival by the PI3 K–Akt signaling pathway. Curr Opin Neurobiol 11:297–305
Chong ZZ, Li F, Maiese K (2005) Activating Akt and the brain’s resources to drive cellular survival and prevent inflammatory injury. Histol Histopathol 20:299–315
Kulich SM, Horbinski C, Patel M et al (2007) 6-Hydroxydopamine induces mitochondrial ERK activation. Free Radic Biol Med 43:372–383
Jiang Z, Yu PH (2005) Involvement of extracellular signal-regulated kinases 1/2 and (phosphoinositide 3-kinase)/Akt signal pathways in acquired resistance against neurotoxin of 6-hydroxydopamine in SH-SY5Y cells following cell-cell interaction with astrocytes. Neuroscience 133:405–411
Smith PD, O’Hare MJ, Park DS (2004) Emerging pathogenic role for cyclin dependent kinases in neurodegeneration. Cell Cycle 3:289–291
Subramaniam S, Unsicker K (2006) Extracellular signal-regulated kinase as an inducer of non-apoptotic neuronal death. Neuroscience 138:1055–1065
Sako K, Fukuhara S, Minami T et al (2008) Angiopoietin-1 induces Kruppel-like factor 2 expression through a phosphoinositide 3-kinase/AKT-dependent activation of myocyte enhancer factor 2. J Biol Chem 284:5592–5601
Acknowledgments
This work was supported by a research grant from Jeju National University in 2007.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, MK., Kim, SC., Kang, JI. et al. 6-Hydroxydopamine-Induced PC12 Cell Death is Mediated by MEF2D Down-regulation. Neurochem Res 36, 223–231 (2011). https://doi.org/10.1007/s11064-010-0309-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-010-0309-x