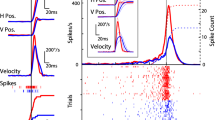
Regularities of spatial expansion of receptive fields (RFs) of visually sensitive neurons upon application of moving visual images were investigated in the cat extrastriate visual cortex (area 21a). The RF size and their spatial infrastructure were first defined by presentation of stationary flashing stimuli, and horizontal and vertical axes (HA and VA, respectively) of classical RFs were determined. Then the lengths of the above axes were carefully measured by spatial scanning of the RFs with moving visual stimuli. It was found that dynamic expansion of the RF sizes was, as a rule, linked to the trajectory of a moving stimulus across the RF. Stimulus motion along the RF HA resulted in significant extension of this axis but not of the VA, while the its motion along the RF VA usually caused extension of only this axis, while another axis underwent negligible changes. These results demonstrate that spatial expansion of the RFs correlates mainly with the trajectory of stimulus motion across the RF. Such an effect probably results from excitation of the neuron under study by the influences coming from adjacent cortical neural networks. Thus, neural circuits localized outside the RF play a decisive role in modulation of the qualitative and quantitative characteristics of classical RFs, hence ensuring precise central processing of incoming visual information.
Similar content being viewed by others
References
H. K. Hartline, “Response of single optic nerve fibers of the vertebrate eye to illumination of the retina,” Am. J. Physiol., 121, No. 2, 400-415 (1938).
H. K. Hartline, “The receptive field of optic nerve fibers,” Am. J. Physiol., 130, No. 3, 690-699 (1940).
D. H. Hubel and T. N. Wiesel, “Receptive fields of single neurons in the cat’s striate cortex,” J. Physiol., 148, No. 3, 574-591 (1959).
D. H. Hubel and T. N. Wiesel. “Receptive fields, binocular interaction, and functional architecture in the cat’s visual cortex,” J. Physiol., 160, No. 1, 106-154 (1962).
A. Angelucci, J. B. Levit, J. S. Walton, et al., “Circuits for local and global integration in primary visual cortex,” J. Neurosci., 22, No. 19, 8633-8646 (2002).
J. Lampi, J. S. Anderson, D. C. Gillespi, and G. H. Ferster, “Predication of orientation selectivity from receptive field architecture in simple cells of cat visual cortex,” Neuron, 30, No. 1, 267-274 (2001).
C. Kyser and P. Konig, “Feature selectivity in area 21a of the cat,” NeuroReport, 17, No. 8, 809-812 (2006).
J. M. G. Tsui and C. C. Pack, “Contrast sensitivity of MT receptive field centers and surrounds,” J. Neurophysiol., 106, No. 4, 1888-1900 (2011).
B. A. Harutiunian-Kozak, G. G. Grigoryan, J. Kozak, et al., “Orientation sensitive properties of visually driven neurons in extrastriate area 21a of cat cortex,” Arch. Ital. Biol., 146, No. 1, 119-130 (2008).
D. K. Khachvankian, J. A. Kozak, A. L. Ghazarian, et al., “Reorganization of receptive fields of cortical field 21a neurons and formation of response to moving visual stimuli,” Neurophysiology, 44, No. 1, 33-41 (2012).
J. J. Pettet and C. D. Gilbert, “Dynamic changes in receptive field size in cat primary visual cortex,” Proc. Natl. Acad. Sci. USA, 89, No. 17, 8366-8370 (1992).
A. Das and C. D. Gilbert, “Receptive fields expansion in adult visual cortex is linked to dynamic changes in strength of cortical connections,” J. Neurophysiol., 74, No. 2, 779-792 (1995).
U. T. Eysel, D. Eyding, and G. Schweigart, “Repetitive optical stimulation elicits fast receptive field changes in mature visual cortex,” NeuroReport, 9, No. 5, 949-954 (1998).
D. K. Khachvankian, B. A. Harutiunian-Kozak, H. R. Aslanian, et al., “Dynamic changes in receptive field sizes of visually sensitive neurons in extrastriate area 21a,” Electron. J. Nat. Sci., 2, No. 23, 42-46 (2014).
P. O. Bishop, W. Kozak, and G. J. Vakkur, “Some quantitative aspects of the cat’s eye: axis and plane reference, visual field co-ordinates and optics,” J. Physiol., 163, No. 3, 466-502 (1962).
R. Fernald and R. Chase, “An improved method for plotting retinal ganglion landmarks and focusing the eyes,” Vision Res., 11, No. 1, 95-96 (1971).
C. Y. Li, P. Ping, Y. X. Zhow, and H. C. Mitzlaff, “Role of the extensive area outside the x-cell receptive field in brightness information transmission,” Vision Res., 31, No. 9, 1529-1540 (1991).
K. Suder, K. Funke, J. Zhao, et al., “Spatial dynamics of receptive fields in cat primary visual cortex related to the temporal structure of thalamocortical feedforward activity,” Exp. Brain. Res., 144, No. 4, 430-444 (2002).
D. Eyding, G. Schweigart, and U. T. Eysel, “Spatiotemporal plasticity of cortical receptive fields in response to repetitive visual stimulation in the adult cat,” Neuroscience, 112, No. 1, 195-215 (2002).
C. D. Gilbert and T. N. Wiesel, “Columnar specificity of intrinsic horizontal and corticotectal connections in cat visual cortex,” J. Neurosci., 9, No. 7, 2432-2442 (1989).
U. Polat and D. Sagi, “Lateral interactions between spatial channels: suppression and facilitation revealed by lateral masking experiments,” Vision Res., 33, No. 7, 993-999 (1993).
A. M. Silito, J. Cudeiro, and H. E. Jones, “Always returning: feedback and sensory processing in visual cortex and thalamus,” Trends Neurosci., 29, No. 6, 307-316 (2006).
L. Schwabe, K. Obermayer, A. Angelucchi, and P. C. Bressloff, “The role of feedback in shaping the extra-classical receptive field of cortical neurons: a recurrent network model,” J. Neurosci., 26, No. 36, 9117-9129 (2006).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Khachvankian, D.K., Ghazaryan, A.L., Harutiunian-Kozak, B.A. et al. Expansion of Visual Receptive Fields in the Extrastriate Visual Cortex: Dependence on the Trajectory of a Moving Stimulus. Neurophysiology 49, 122–129 (2017). https://doi.org/10.1007/s11062-017-9640-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11062-017-9640-z