Abstract
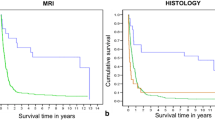
Childhood brain tumors show great histological variability. The goal of this retrospective study was to assess the diagnostic accuracy of multimodal MR imaging (diffusion, perfusion, MR spectroscopy) in the distinction of pediatric brain tumor grades and types. Seventy-six patients (range 1 month to 18 years) with brain tumors underwent multimodal MR imaging. Tumors were categorized by grade (I–IV) and by histological type (A–H). Multivariate statistical analysis was performed to evaluate the diagnostic accuracy of single and combined MR modalities, and of single imaging parameters to distinguish the different groups. The highest diagnostic accuracy for tumor grading was obtained with diffusion–perfusion (73.24 %) and for tumor typing with diffusion–perfusion–MR spectroscopy (55.76 %). The best diagnostic accuracy was obtained for tumor grading in I and IV and for tumor typing in embryonal tumor and pilocytic astrocytoma. Poor accuracy was seen in other grades and types. ADC and rADC were the best parameters for tumor grading and typing followed by choline level with an intermediate echo time, CBV for grading and Tmax for typing. Multiparametric MR imaging can be accurate in determining tumor grades (primarily grades I and IV) and types (mainly pilocytic astrocytomas and embryonal tumors) in children.

Similar content being viewed by others
References
Koob M, Girard N (2014) Cerebral tumors: specific features in children. Diagn Interv Imaging 95(10):965–983. doi:10.1016/j.diii.2014.06.017
Porto L, Jurcoane A, Schwabe D, Hattingen E (2014) Conventional magnetic resonance imaging in the differentiation between high and low-grade brain tumours in paediatric patients. Eur J Paediatr Neurol 18(1):25–29. doi:10.1016/j.ejpn.2013.07.004
Sugahara T, Korogi Y, Kochi M, Ikushima I, Shigematu Y, Hirai T, Okuda T, Liang L, Ge Y, Komohara Y, Ushio Y, Takahashi M (1999) Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. JMRI 9(1):53–60
Tzika AA, Astrakas LG, Zarifi MK, Petridou N, Young-Poussaint T, Goumnerova L, Zurakowski D, Anthony DC, Black PM (2003) Multiparametric MR assessment of pediatric brain tumors. Neuroradiology 45(1):1–10
Tzika AA, Vajapeyam S, Barnes PD (1997) Multivoxel proton MR spectroscopy and hemodynamic MR imaging of childhood brain tumors: preliminary observations. AJNR 18(2):203–218
Brandao LA, Poussaint TY (2013) Pediatric brain tumors. Neuroimaging Clin N Am 23(3):499–525. doi:10.1016/j.nic.2013.03.003
Panigrahy A, Bluml S (2009) Neuroimaging of pediatric brain tumors: from basic to advanced magnetic resonance imaging (MRI). J Child Neurol 24(11):1343–1365. doi:10.1177/0883073809342129
Poretti A, Meoded A, Huisman TA (2012) Neuroimaging of pediatric posterior fossa tumors including review of the literature. J Magn Reson Imaging 35(1):32–47. doi:10.1002/jmri.22722
Jaremko JL, Jans LB, Coleman LT, Ditchfield MR (2010) Value and limitations of diffusion-weighted imaging in grading and diagnosis of pediatric posterior fossa tumors. AJNR 31(9):1613–1616. doi:10.3174/ajnr.A2155
Panigrahy A, Krieger MD, Gonzalez-Gomez I, Liu X, McComb JG, Finlay JL, Nelson MD Jr, Gilles FH, Bluml S (2006) Quantitative short echo time 1H-MR spectroscopy of untreated pediatric brain tumors: preoperative diagnosis and characterization. AJNR 27(3):560–572
Porto L, Jurcoane A, Schwabe D, Kieslich M, Hattingen E (2013) Differentiation between high and low grade tumours in paediatric patients by using apparent diffusion coefficients. Eur J Paediatr Neurol 17(3):302–307. doi:10.1016/j.ejpn.2012.12.002
Schneider JF, Confort-Gouny S, Viola A, Le Fur Y, Viout P, Bennathan M, Chapon F, Figarella-Branger D, Cozzone P, Girard N (2007) Multiparametric differentiation of posterior fossa tumors in children using diffusion-weighted imaging and short echo-time 1H-MR spectroscopy. J Magn Reson Imaging 26(6):1390–1398. doi:10.1002/jmri.21185
Welker K, Boxerman J, Kalnin A, Kaufmann T, Shiroishi M, Wintermark M, American Society of Functional Neuroradiology MRPS, Practice Subcommittee of the ACPC (2015) ASFNR recommendations for clinical performance of MR dynamic susceptibility contrast perfusion imaging of the brain. AJNR 36(6):E41–E51. doi:10.3174/ajnr.A4341
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114(2):97–109
Girard N, Gouny SC, Viola A, Le Fur Y, Viout P, Chaumoitre K, D’Ercole C, Gire C, Figarella-Branger D, Cozzone PJ (2006) Assessment of normal fetal brain maturation in utero by proton magnetic resonance spectroscopy. Magn Reson Med 56(4):768–775. doi:10.1002/mrm.21017
Fellah S, Caudal D, De Paula AM, Dory-Lautrec P, Figarella-Branger D, Chinot O, Metellus P, Cozzone PJ, Confort-Gouny S, Ghattas B, Callot V, Girard N (2013) Multimodal MR imaging (diffusion, perfusion, and spectroscopy): is it possible to distinguish oligodendroglial tumor grade and 1p/19q codeletion in the pretherapeutic diagnosis? AJNR 34(7):1326–1333. doi:10.3174/ajnr.A3352
Wu O, Ostergaard L, Weisskoff RM, Benner T, Rosen BR, Sorensen AG (2003) Tracer arrival timing-insensitive technique for estimating flow in MR perfusion-weighted imaging using singular value decomposition with a block-circulant deconvolution matrix. Magn Reson Med 50(1):164–174. doi:10.1002/mrm.10522
Lobel U, Sedlacik J, Reddick WE, Kocak M, Ji Q, Broniscer A, Hillenbrand CM, Patay Z (2011) Quantitative diffusion-weighted and dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging analysis of T2 hypointense lesion components in pediatric diffuse intrinsic pontine glioma. AJNR 32(2):315–322
Toh CH, Wei KC, Chang CN, Ng SH, Wong HF (2013) Differentiation of primary central nervous system lymphomas and glioblastomas: comparisons of diagnostic performance of dynamic susceptibility contrast-enhanced perfusion MR imaging without and with contrast-leakage correction. AJNR 34(6):1145–1149. doi:10.3174/ajnr.A3383
Tsolaki E, Kousi E, Svolos P, Kapsalaki E, Theodorou K, Kappas C, Tsougos I (2014) Clinical decision support systems for brain tumor characterization using advanced magnetic resonance imaging techniques. World J Radiol 6(4):72–81. doi:10.4329/wjr.v6.i4.72
Breinman LFJ, Olsen RA, Stone CJ (1984) Classification and regression trees. Wadsworth International Group, Belmont
Rumboldt Z, Camacho DL, Lake D, Welsh CT, Castillo M (2006) Apparent diffusion coefficients for differentiation of cerebellar tumors in children. AJNR 27(6):1362–1369
Gimi B, Cederberg K, Derinkuyu B, Gargan L, Koral KM, Bowers DC, Koral K (2012) Utility of apparent diffusion coefficient ratios in distinguishing common pediatric cerebellar tumors. Acad Radiol 19(7):794–800. doi:10.1016/j.acra.2012.03.004
Raschke F, Davies NP, Wilson M, Peet AC, Howe FA (2013) Classification of single-voxel 1H spectra of childhood cerebellar tumors using LCModel and whole tissue representations. Magn Reson Med 70(1):1–6. doi:10.1002/mrm.24461
Vicente J, Fuster-Garcia E, Tortajada S, Garcia-Gomez JM, Davies N, Natarajan K, Wilson M, Grundy RG, Wesseling P, Monleon D, Celda B, Robles M, Peet AC (2013) Accurate classification of childhood brain tumours by in vivo (1)H MRS—a multi-centre study. Eur J Cancer 49(3):658–667. doi:10.1016/j.ejca.2012.09.003
Shiroishi MS, Panigrahy A, Moore KR, Nelson MD Jr, Gilles FH, Gonzalez-Gomez I, Bluml S (2015) Combined MRI and MRS improves pre-therapeutic diagnoses of pediatric brain tumors over MRI alone. Neuroradiology 57(9):951–956. doi:10.1007/s00234-015-1553-1
Al-Okaili RN, Krejza J, Woo JH, Wolf RL, O’Rourke DM, Judy KD, Poptani H, Melhem ER (2007) Intraaxial brain masses: MR imaging-based diagnostic strategy–initial experience. Radiology 243(2):539–550
Davies NP, Wilson M, Harris LM, Natarajan K, Lateef S, Macpherson L, Sgouros S, Grundy RG, Arvanitis TN, Peet AC (2008) Identification and characterisation of childhood cerebellar tumours by in vivo proton MRS. NMR Biomed 21(8):908–918
Kitis O, Altay A, Calli C, Yunten N, Yurtseven T (2005) Minimum apparent coefficients in the evaluation of brain tumors. Eur J Radiol 55(3):393–400
Lee EJ, Lee SK, Agid R, Bae JM, Keller A, Terbrugge K (2008) Preoperative grading of presumptive low-grade astrocytomas on MR imaging: diagnostic value of minimum apparent diffusion coefficient. AJNR 29(10):1872–1877
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
N Girard has consulting with Olea Medical.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11060_2015_2042_MOESM1_ESM.tif
Example of ROI placement in a posterior fossa medulloblastoma. The ROI positioned on the CBV map over the solid part of the tumor and the control ROI in left cerebellar grey matter are automatically overlaid on the other perfusion (corrected CBV, K2, MTT, TTP, tMIP) and ADC maps, as well as on T2WI and post-GBCAs T1WI. This prevents the inclusion of macroscopic vessels within the ROI. The perfusion curve is at the top left. Supplementary material 1 (TIFF 5605 kb)
11060_2015_2042_MOESM2_ESM.tif
Diagnostic accuracy (%) as a function of the number of best-performing parameters successively added for the determination of tumour grading. Supplementary material 2 (TIFF 892 kb)
11060_2015_2042_MOESM3_ESM.tif
Diagnostic accuracy (%) as a function of the number of best-performing parameters successively added for the determination of typing. Supplementary material 3 (TIFF 892 kb)
Rights and permissions
About this article
Cite this article
Koob, M., Girard, N., Ghattas, B. et al. The diagnostic accuracy of multiparametric MRI to determine pediatric brain tumor grades and types. J Neurooncol 127, 345–353 (2016). https://doi.org/10.1007/s11060-015-2042-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-2042-4