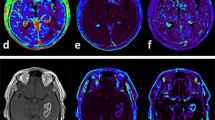
Abstract
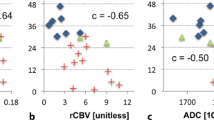
Patients with high-grade gliomas usually have heterogeneous response to surgery and chemoirradiation. The objectives of this study were (1) to evaluate serial changes in tumor volume and perfusion imaging parameters and (2) to determine the value of these data in predicting overall survival (OS). Twenty-nine patients with World Health Organization grades III and IV gliomas underwent magnetic resonance (MR) and computed tomography (CT) perfusion examinations before surgery, and 1, 3, 6, 9, and 12 months after radiotherapy. Serial measurements of tumor volumes and perfusion parameters were evaluated by receiver operating characteristic analysis, Cox proportional hazards regression, and Kaplan–Meier survival analysis to determine their values in predicting OS. Higher trends in blood flow (BF), blood volume (BV), and permeability-surface area product in the contrast-enhancing lesions (CEL) and the non-enhancing lesions (NEL) were found in patients with OS < 18 months compared to those with OS ≥ 18 months, and these values were significant at selected time points (P < 0.05). Only CT perfusion parameters yielded sensitivities and specificities of ≥70 % in predicting 18 and 24 months OS. Pre-surgery BF in the NEL and BV in the CEL and NEL 3 months after radiotherapy had sensitivities and specificities >80 % in predicting 24 months OS in patients with grade IV gliomas. Our study indicated that CT perfusion parameters were predictive of survival and could be useful in assessing early response and in selecting adjuvant treatment to prolong survival if verified in a larger cohort of patients.



Similar content being viewed by others
References
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359:492–507
Curran WJ, Scott CB, Horton J et al (1993) Recursive partitioning analysis of prognostic factors in three radiation therapy oncology group malignant glioma trials. J Natl Cancer Inst 85:704–710
Weller M, Stupp R, Hegi ME et al (2012) Personalized care in neuro-oncology coming of age: why we need MGMT and 1p/19q testing for malignant glioma patients in clinical practice. Neuro Oncol 14(suppl iv):100–108
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972
Essig M, Anzalone N, Combs SE et al (2012) MR imaging of neoplastic central nervous system lesions: Review and recommendations for current practice. Am J Neuroradiol 33:803–817
Cha S (2006) Update on brain tumor imaging: from anatomy to physiology. Am J Neuroradiol 27:475–487
Jain R, Ellika SK, Scarpace L et al (2008) Quantitative estimation of permeability surface-area product in astroglial brain tumors using perfusion CT and correlation with histopathologic grade. Am J Neuroradiol 29:694–700
Weber MA, Henze M, Tüttenberg J et al (2010) Biopsy targeting gliomas: do functional imaging techniques identify similar target areas? Invest Radiol 45:755–768
Jain R, Gutierrez J, Narang J et al (2011) In vivo correlation of tumor blood volume and permeability with histologic and molecular angiogenic markers in gliomas. Am J Neuroradiol 32:388–394
Hu LS, Eschbacher JM, Dueck AC et al (2012) Correlations between perfusion MR imaging cerebral blood volume, microvessel quantification, and clinical outcome using stereotactic analysis in recurrent high-grade glioma. Am J Neuroradiol 33:69–76
Jain R, Narang J, Griffith B et al (2013) Prognostic vascular imaging biomarkers in high-grade gliomas: tumor permeability as an adjunct to blood volume estimates. Acad Radiol 20:478–485
Shankar JJ, Woulfe J, Silva VD, Nguyen TB (2013) Evaluation of perfusion CT in grading and prognostication of high-grade gliomas at diagnosis: a pilot study. AJR Am J Roentgenol 200:W504–W509
Law M, Young RJ, Babb JS et al (2008) Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 247:490–498
Bisdas S, Kirkpatrick M, Giglio P, Welsh C, Spampinato MV, Rumboldt Z (2009) Cerebral blood volume measurements by perfusion-weighted MR imaging in gliomas: ready for prime time in predicting short-term outcome and recurrent disease? Am J Neuroradiol 30:681–688
Galbán CJ, Chenevert TL, Meyer CR et al (2011) Prospective analysis of parametric response map-derived MRI biomarkers: Identification of early and distinct glioma response patterns not predicted by standard radiographic assessment. Clin Cancer Res 17:4751–4760
Jain R (2011) Perfusion CT imaging of brain tumors: an overview. AJNR Am J Neuroradiol 32:1570–1577
Ding B, Ling HW, Chen KM, Jiang H, Zhu YB (2006) Comparison of cerebral blood volume and permeability in preoperative grading of intracranial glioma using CT perfusion imaging. Neuroradiology 48:773–781
Fainardi E, Di Biase F, Borrelli M et al (2010) Potential role of CT perfusion parameters in the identification of solitary intra-axial brain tumor grading. Acta Neurochir Suppl 106:283–287
Ellika SK, Jain R, Patel SC et al (2007) Role of perfusion CT in glioma grading and comparison with conventional MR imaging features. Am J Neuroradiol 28:1981–1987
Jain R, Narang J, Schultz L et al (2011) Permeability estimates in histopathology-proved treatment-induced necrosis using perfusion CT: Can these add to other perfusion parameters in differentiating from recurrent/progressive tumors? Am J Neuroradiol 32:658–663
Vidiri A, Guerrisi A, Pinzi V et al (2012) Perfusion Computed Tomography (PCT) adopting different perfusion metrics: recurrence of brain metastasis or radiation necrosis? Eur J Radiol 81:1246–1252
Herman JG, Graff JR, Myöhänen S, Nelkin BD, Baylin SB (1996) Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA 93:9821–9826
Hartmann C, Meyer J, Balss J et al (2009) Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol 118:469–474
Yeung TPC, Yartsev Y, Lee TY et al (2014) Relationship of computed tomography perfusion and positron emission tomography to tumour progression in malignant glioma. J Med Radiat Sci 61:4–13
Yeung TPC, Yartsev S, Bauman G, He W, Fainardi E, Lee TY (2013) The effect of scan duration on the measurement of perfusion parameters in CT perfusion studies of brain tumors. Acad Radiol 20:59–65
Pieper S, Lorensen B, Schroeder W, Kikinis R (2006) The NA-MIC Kit: ITK, VTK, pipelines, grids and 3D slicer as an open platform for the medical image computing community. In: Proceedings of the 3rd IEEE international symposium biomedical imaging: From Nano Macro, vol 1, pp 698–701
Rizopoulos D (2010) JM: an R package for the joint modelling of longitudinal and time-to-event data. J Stat Softw 35:1–33
Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT (2007) Angiogenesis in brain tumours. Nat Rev Neurosci 8:610–622
Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM (2010) Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest 120:694–705
Milano MT, Okunieff P, Donatello RS et al (2010) Patterns and timing of recurrence after temozolomide-based chemoradiation for glioblastoma. Int J Radiat Oncol Biol Phys 78:1147–1155
Vöglein J, Tüttenberg J, Weimer M et al (2011) Treatment monitoring in gliomas: comparison of dynamic susceptibility-weighted contrast-enhanced and spectroscopic MRI techniques for identifying treatment failure. Invest Radiol 46:390–400
Mangla R, Singh G, Ziegelitz D et al (2010) Changes in relative cerebral blood volume 1 month after radiation-temozolomide therapy can help predict overall survival in patients with glioblastoma. Radiology 256:575–584
van den Bent MJ, Vogelbaum MA, Wen PY, Macdonald DR, Chang SM (2009) End point assessment in gliomas: novel treatments limit usefulness of classical Macdonald's Criteria. J Clin Oncol 27:2905–2908
Finn MA, Blumenthal DT, Salzman KL et al (2007) Transient postictal MRI changes in patients with brain tumors may mimic disease progression. Surg Neurol 67:246–250
Ulmer S, Braga TA, Barker FG 2nd et al (2006) Clinical and radiographic features of peritumoral infarction following resection of glioblastoma. Neurology 67:1668–1670
Kumar AJ, Leeds NE, Fuller GN et al (2000) Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 217:377–384
Oh J, Cha S, Aiken AH et al (2005) Quantitative apparent diffusion coefficients and T2 relaxation times in characterizing contrast enhancing brain tumors and regions of peritumoral edema. J Magn Reson Imaging 21:701–708
Hattingen E, Jurcoane A, Daneshvar K et al (2013) Quantitative T2 mapping of recurrent glioblastoma under Bevacizumab improves monitoring for non-enhancing tumor progression and predicts overall survival. Neuro Oncol 15:1395–1404
Li Y, Lupo JM, Polley MY et al (2011) Serial analysis of imaging parameters in patients with newly diagnosed glioblastoma multiforme. Neuro Oncol 13:546–557
Acknowledgments
This project was funded by The Project of Emilia-Romagna region on Neuro-Oncology (PERNO) study group, the Canadian Institutes of Health Research (CIHR), and the CIHR Strategic Training Program in Cancer Research and Technology Transfer.
Funding
Ting-Yim Lee licenses CT Perfusion software to and receives funding from GE Healthcare.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
A complete list of the members of the PERNO study group appears in the “Appendix”.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11060_2015_1766_MOESM1_ESM.tif
Supplementary Figure S1. Serial changes in mean volumes (top row), blood flow (BF, second row), blood volume (BV, third row), and permeability-surface area product (PS, bottom row) in the contrast-enhancing lesions (CEL) of patients stratified by a 12 and b 24 months (mo) overall survival (OS). Horizontal line connects significant changes between two time points of the same group. Dotted box encloses a significant difference between the groups at a particular time point. Error bar represents one standard deviation (TIFF 159 kb)
11060_2015_1766_MOESM2_ESM.tif
Supplemeantary Figure S2. Serial changes in mean volumes (top row), blood flow (BF, second row), blood volume (BV, third row), and permeability-surface area product (PS, bottom row) in the non-enhancing lesion (NEL) of patients stratified by a 12 and b 24 months (mo) overall survival (OS). Horizontal line connects significant changes between two time points of the same group. Dotted box encloses a significant difference between the groups at a particular time point. Error bar represents one standard deviation (TIFF 155 kb)
Appendix
Appendix
Steering committee
Baruzzi A. (Chair), Albani F., Calbucci F., D'Alessandro R., Michelucci R. (IRCCS Institute of Neurological Sciences, Bologna, Italy), Brandes A. (Department of Medical Oncology, Bellaria-Maggiore Hospitals, Bologna, Italy), Eusebi V. (Department of Hematology and Oncological Sciences “L. & A. Seràgnoli”, Section of Anatomic Pathology at Bellaria Hospital, Bologna, Italy), Ceruti S., Fainardi E., Tamarozzi R. (Neuroradiology Unit, Department of Neurosciences and Rehabilitation, S. Anna Hospital, Ferrara, Italy), Emiliani E. (Istituto Oncologico Romagnolo, Department of Medical Oncology, Santa Maria delle Croci Hospital, Ravenna, Italy), Cavallo M. (Division of Neurosurgery, Department of Neurosciences and Rehabilitation, S. Anna Hospital, Ferrara, Italy).
Executive committee
Franceschi E., Tosoni A. (Department of Medical Oncology, Bellaria-Maggiore Hospitals, Bologna, Italy), Cavallo M. (Division of Neurosurgery, Department of Neurosciences and Rehabilitation, S. Anna Hospital, Ferrara, Italy), Fiorica F. (Department of Radiation Oncology, S. Anna Hospital, Ferrara, Italy), Valentini A. (Division of Neurosurgery, Nuovo Ospedale Civile S. Agostino-Estense, Baggiovara, Modena, Italy), Depenni R. (Department of Oncology, Policlinico di Modena, Italy), Mucciarini C. (Department of Oncology, Ramazzini Hospital, Carpi, Modena, Italy), Crisi G. (Department of Neuroradiology, Maggiore Hospital, Parma, Italy), Sasso E. (Department of Neurological Sciences, Maggiore Hospital, Parma, Italy), Biasini C., Cavanna L. (Department of Oncology and Hematology, Guglielmo da Saliceto Hospital, Piacenza, Italy), Guidetti D. (Department of Neurology, Guglielmo da Saliceto Hospital, Piacenza, Italy), Marcello N., Pisanello A. (Department of Neurology, Istituto in tecnologie avanzate e modelli assistenziali in oncologia, IRCCS, S. Maria Nuova Hospital, Reggio Emilia, Italy), Cremonini A.M., Guiducci G. (Division of Neurosurgery, M. Bufalini Hospital, Cesena, Italy).
Registry Coordination Office: de Pasqua S., Testoni S. (IRCCS Institute of Neurological Sciences, Bologna, Italy).
Participants
Agati R., Ambrosetto G., Bacci A., Baldin E., Baldrati A., Barbieri E., Bartolini S., Bellavista E., Bisulli F., Bonora E., Bunkheila F., Carelli V., Crisci M., Dall'Occa P., Ferro S., Franceschi C., Frezza G., Grasso V., Leonardi M., Morandi L., Mostacci B., Palandri G., Pasini E., Pastore Trossello M., Poggi R., Riguzzi P., Rinaldi R., Rizzi S., Romeo G., Spagnolli F., Tinuper P., Trocino C. (Bologna), Dall'Agata M., Frattarelli M., Gentili G., Giovannini A., Iorio P., Pasquini U., Galletti G., Guidi C., Neri W., Patuelli A., Strumia S. (Forlì-Cesena), Faedi M. (IRCCS Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori), Casmiro M., Gamboni A., Rasi F. (Faenza R.A.), Cruciani G. (Lugo, RA), Cenni P., Dazzi C., Guidi A.R., Zumaglini F. (Ravenna), Amadori A., Pasini G., Pasquinelli M., Pasquini E., Polselli A., Ravasio A., Viti B. (Rimini), Sintini M. (Cattolica, RN), Ariatti A., Bertolini F., Bigliardi G., Carpeggiani P., Cavalleri F., Meletti S., Nichelli P., Pettorelli E., Pinna G., Zunarelli E. (Modena), Artioli F., Bernardini I., Costa M., Greco G., Guerzoni R., Stucchi C. (Carpi M.O.), Iaccarino C., Ragazzi M., Rizzi R., Zuccoli G. (Istituto di Ricovero e Cura a Carattere Scientifico, Reggio Emilia), Api P., Cartei F., Colella M., Fallica E., Farneti M., Frassoldati A., Granieri E., Latini F., Monetti C., Saletti A., Schivalocchi R., Sarubbo S., Seraceni S., Tola M.R., Urbini B., Zini G. (Ferrara), Giorgi C., Montanari E. (Fidenza P.R.), Cerasti D., Crafa P., Dascola I., Florindo I., Giombelli E., Mazza S., Ramponi V., Servadei F., Silini E.M., Torelli P. (Parma), Immovilli P., Morelli N., Vanzo C. (Piacenza), Nobile C. (Padova).
Rights and permissions
About this article
Cite this article
Yeung, T.P.C., Wang, Y., He, W. et al. Survival prediction in high-grade gliomas using CT perfusion imaging. J Neurooncol 123, 93–102 (2015). https://doi.org/10.1007/s11060-015-1766-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-1766-5