Abstract
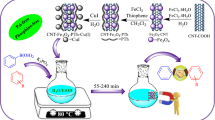
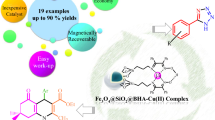
The development of heterogenization of copper nanoparticles on conductive supports is very challenging and has received much attention. Here, we synthesize a practical, efficient, and inexpensive heterogeneous catalyst to grow stable metallic copper(II) nanoparticles on the surface of magnetic carbon nanotube (Fe3O4–CNT) catalyst support physically functionalized with triethanolamine (TEA) that acts as a low-cost and non-toxic ligand to capture the copper nanoparticles [Fe3O4–CNT–TEA–Cu(II)]. The as-prepared heterogeneous catalyst was characterized by different techniques, such as Fourier transform infrared spectroscopy, energy-dispersive X-ray spectroscopy, thermogravimetric analysis, vibrating sample magnetometer, X-ray diffraction patterns, field-emission scanning electron microscopy, and atomic absorption spectroscopy analysis. The catalytic behavior of Fe3O4–CNT–TEA–Cu(II) was investigated in the preparation of 5-substituted 1H-tetrazole derivatives via one-pot, three-component reaction between aromatic aldehydes, hydroxylamine, and sodium azide. The low catalyst loading, wide substrate scope, use of inexpensive materials, simple separation of the catalyst from the reaction mixture by an external magnet, short reaction times, easy workup, affordability, and superb yield are some advantages of this protocol.
Graphical abstract













Similar content being viewed by others
References
De Volde MF, Tawfick SH, Baughman RH, Hart AJ (2013) Carbon nanotubes: present and future commercial applications. Science 339:535–539. https://doi.org/10.1126/science.1222453
Tsapenko AP, Goldt AE, Shulga E, Popov ZI, Maslakov KI, Anisimov AS, Sorokin PB, Nasibulin AG (2018) Highly conductive and transparent films of HAuCl4-doped single-walled carbon nanotubes for flexible applications. Carbon 130:448–457. https://doi.org/10.1016/j.carbon.2018.01.016
Qi P, Vermesh O, Grecu M, Javey A, Wang Q, Dai H, Peng S, Cho K (2003) Toward large arrays of multiplex functionalized carbon nanotube sensors for highly sensitive and selective molecular detection. Nano Lett 3:347–351. https://doi.org/10.1021/nl034010k
Dalton AB, Collins S, Munoz E, Razal JM, Ebron VH, Ferraris JP, Coleman JN, Kim BG, Baughman RH (2003) Super-tough carbon-nanotube fibres. Nature 423:703. https://doi.org/10.1038/423703a
Zhang Q, Huang JQ, Qian WZ, Zhang YY, Wei F (2013) The road for nanomaterials industry: a review of carbon nanotube production, post-treatment, and bulk applications for composites and energy storage. Small 9:1237–1265. https://doi.org/10.1002/smll.201203252
Kour G, Gupta M, Vishwanathan B, Thirunavukkarasu K (2016) (Cu/NCNTs): a new high temperature technique to prepare a recyclable nanocatalyst for four component pyridine derivative synthesis and nitroarenes reduction. New J Chem 40:8535–8542. https://doi.org/10.1039/C6NJ01464J
Zhai Y, Dou Y, Zhao D, Fulvio PF, Mayes RT, Dai S (2011) Carbon materials for chemical capacitive energy storage. Adv Mater 23:4828–4850. https://doi.org/10.1002/adma.201100984
Guo DJ, Li HL (2006) Electrocatalytic oxidation of methanol on Pt modified single-walled carbon nanotubes. J Power Sources 160:44–49. https://doi.org/10.1016/j.jpowsour.2006.01.026
Kim E, Jeong HS, Kim BM (2014) Studies on the functionalization of MWNTs and their application as a recyclable catalyst for CC bond coupling reactions. Catal Commun 46:71–74. https://doi.org/10.1016/j.catcom.2013.11.028
Karousis N, Tagmatarchis N, Tasis D (2010) Current progress on the chemical modification of carbon nanotubes. Chem Rev 110:5366–5397. https://doi.org/10.1021/cr100018g
Shaabani A, Afshari R, Hooshmand SE, Tabatabaei AT, Hajishaabanha F (2016) Copper supported on MWCNT-guanidine acetic acid@Fe3O4: synthesis, characterization and application as a novel multi-task nanocatalyst for preparation of triazoles and bis (indolyl) methanes in water. RSC Adv 6:18113–18125. https://doi.org/10.1039/C5RA23294E
Bacsa RR, Serp P (2011) Decorated (coated) carbon nanotubes: (X/CNTs). In: Carbon meta-nanotubes: synthesis, properties and applications, pp 163–221. https://doi.org/10.1002/9781119954743.ch4
Khanna P, Gaikwad S, Adhyapak P, Singh N, Marimuthu R (2007) Synthesis and characterization of copper nanoparticles. Mater Lett 61:4711–4714. https://doi.org/10.1016/j.matlet.2007.03.014
Seo JY, Kang HW, Jung DS, Lee HM, Park SB (2013) One-step synthesis of copper nanoparticles embedded in carbon composites. Mater Res Bull 48:1484–1489. https://doi.org/10.1021/acs.langmuir.7b02900
Xia X, Xie C, Cai S, Yang Z, Yang X (2006) Corrosion characteristics of copper microparticles and copper nanoparticles in distilled water. Corros Sci 48:3924–3932. https://doi.org/10.1016/j.corsci.2006.04.007
Yanase A, Komiyama H (1991) Real-time optical observation of morphological change of small supported copper particles during redox treatments. Surf Sci 248:20–26. https://doi.org/10.1016/0039-6028(91)90057-Y
Hafez IH, Berber MR, Fujigaya T, Nakashima N (2017) High electronic conductivity and air stability of ultrasmall copper–metal nanoparticles supported on pyridine-based polybenzimidazole carbon nanotube composite. ChemCatChem 9:4282–4286. https://doi.org/10.1002/cctc.201700921
Zhang HX, Siegert U, Liu R, Cai WB (2009) Facile fabrication of ultrafine copper nanoparticles in organic solvent. Nanoscale Res Lett 4:705. https://doi.org/10.1007/s11671-009-9301-2
Cioffi N, Torsi L, Ditaranto N, Sabbatini L, Zambonin PG, Tantillo G, Ghibelli L, D’Alessio M, Bleve-Zacheo T, Traversa E (2004) Antifungal activity of polymer-based copper nanocomposite coatings. Appl Phys Lett 85:2417–2419. https://doi.org/10.1063/1.1794381
Shaabani A, Afshari R (2018) Magnetic Ugi-functionalized graphene oxide complexed with copper nanoparticles: efficient catalyst toward Ullman coupling reaction in deep eutectic solvents. J Colloid Interface Sci 510:384–394. https://doi.org/10.1016/j.jcis.2017.09.089
Elhamifar D, Ardeshirfard H (2017) Phenyl and ionic liquid based bifunctional periodic mesoporous organosilica supported copper: an efficient nanocatalyst for clean production of polyhydroquinolines. J Colloid Interface Sci 505:1177–1184. https://doi.org/10.1016/j.jcis.2017.07.010
Agrahari B, Layek S, Ganguly R, Pathak DD (2018) Synthesis and crystal structures of salen-type Cu(ii) and Ni(ii) Schiff base complexes: application in [3+2]-cycloaddition and A3-coupling reactions. New J Chem 42:13754–13762. https://doi.org/10.1039/C8NJ01718B
Feng J, He Y, Liu Y, Du Y, Li D (2015) Supported catalysts based on layered double hydroxides for catalytic oxidation and hydrogenation: general functionality and promising application prospects. Chem Soc Rev 44:5291–5319. https://doi.org/10.1039/C5CS00268K
Zou Y, Wang P, Yao W, Wang X, Liu Y, Yang D, Wang L, Hou J, Alsaedi A, Hayat T (2017) Synergistic immobilization of UO22+ by novel graphitic carbon nitride@ layered double hydroxide nanocomposites from wastewater. Chem Eng J 330:573–584. https://doi.org/10.1016/j.cej.2017.07.135
Wattanathana W, Nootsuwan N, Veranitisagul C, Koonsaeng N, Laosiripojana N, Laobuthee A (2015) Simple cerium-triethanolamine complex: synthesis, characterization, thermal decomposition and its application to prepare ceria support for platinum catalysts used in methane steam reforming. J Mol Struct 1089:9–15. https://doi.org/10.1016/j.molstruc.2015.02.010
Lommens P, Tack P, Vander Elst L, Van Driessche I, Vincze L, Sinnaeve D (2018) Spectroscopy as a tool to detect multinuclear Cu(II)–triethanolamine complexes in aqueous solution. Dalton Trans 47:3755–3763. https://doi.org/10.1039/C7DT04146B
Karadag A, Yilmaz VT, Thoene C (2001) Thoene, Di-and triethanolamine complexes of Co(II), Ni(II), Cu(II) and Zn(II) with thiocyanate: synthesis, spectral and thermal studies. Crystal structure of dimeric Cu(II) complex with deprotonated diethanolamine, [Cu2(μ-dea)2(NCS)2]. Polyhedron 20:635–641. https://doi.org/10.1016/S0277-5387(01)00720-3
Shiri L, Zarei S, Kazemi M, Sheikh D (2018) Sulfuric acid heterogenized on magnetic Fe3O4 nanoparticles: a new and efficient magnetically reusable catalyst for condensation reactions. Appl Organomet Chem 32:e3938. https://doi.org/10.1002/aoc.3938
Veisi H, Pirhayati M, Kakanejadifard A (2017) Immobilization of palladium nanoparticles on ionic liquid-triethylammonium chloride functionalized magnetic nanoparticles: as a magnetically separable, stable and recyclable catalyst for Suzuki–Miyaura cross-coupling reactions. Tetrahedron Lett 58:4269–4276. https://doi.org/10.1016/j.tetlet.2017.09.078
Mohammadinezhad A, Akhlaghinia B (2017) Fe3O4@Boehmite-NH2-CoII NPs: an inexpensive and highly efficient heterogeneous magnetic nanocatalyst for the Suzuki-Miyaura and Heck-Mizoroki cross-coupling reactions. Green Chem 19:5625–5641. https://doi.org/10.1039/C7GC02647A
Fan GY, Huang WJ (2015) Solvent-free hydrogenation of nitrobenzene catalyzed by magnetically recoverable Pt deposited on multiwalled carbon nanotubes. Synth React Inorg Met-Org Nano-Met Chem 45:819–1825. https://doi.org/10.1080/15533174.2013.872139
Kappe C, Zhu J (2005) Multicomponent reactions. Wiley-VCH, Weinheim
Cioc RC, Ruijter E, Orru RV (2014) Multicomponent reactions: advanced tools for sustainable organic synthesis. Green Chem 16:2958–2975. https://doi.org/10.1039/C4GC00013G
Brauch S, Van Berkel SS, Westermann B (2013) Higher-order multicomponent reactions: beyond four reactants. Chem Soc Rev 42:4948–4962. https://doi.org/10.1039/C3CS35505E
Tahmasbi B, Ghorbani-Choghamarani A (2017) First report of the direct supporting of palladium–arginine complex on boehmite nanoparticles and application in the synthesis of 5-substituted tetrazoles. Appl Organometal Chem 31:e3644. https://doi.org/10.1002/aoc.3644
Taghavi F, Gholizadeh M, Saljooghi AS, Ramezani M (2017) Cu(ii) immobilized on Fe3O4@APTMS-DFX nanoparticles: an efficient catalyst for the synthesis of 5-substituted 1H-tetrazoles with cytotoxic activity. MedChemComm 8:1953–1964. https://doi.org/10.1039/C7MD00302A
Prajapti SK, Nagarsenkar A, Babu BN (2014) An efficient synthesis of 5-substituted 1H-tetrazoles via B(C6F5)3 catalyzed [3 + 2] cycloaddition of nitriles and sodium azide. Tetrahedron Lett 55:3507–3510. https://doi.org/10.1016/j.tetlet.2014.04.089
Abrishami F, Ebrahimikia M, Rafiee F (2015) Synthesis of 5-substituted 1H-tetrazoles using a recyclable heterogeneous nanonickel ferrite catalyst. Appl Organometal Chem 29:730–735. https://doi.org/10.1002/aoc.3358
Gutmann B, Roduit JP, Roberge D, Kappe CO (2010) Synthesis of 5-substituted 1H-tetrazoles from nitriles and hydrazoic acid by using a safe and scalable high-temperature microreactor approach. Angew Chem 122:7255–7259. https://doi.org/10.1002/anie.201003733
Joshi SM, Mane RB, Pulagam KR, Gomez-Vallejo V, Llop J, Rode C (2017) The microwave-assisted synthesis of 5-substituted 1H-tetrazoles via [3 + 2] cycloaddition over a heterogeneous Cu-based catalyst: application to the preparation of 13N-labelled tetrazoles. New J Chem 41:8084–8091. https://doi.org/10.1039/C7NJ00568G
Sridhar M, Mallu KKR, Jillella R, Godala KR, Beeram CR, Chinthala N (2013) One-step synthesis of 5-substituted 1H-tetrazoles from an aldehyde by reaction with acetohydroxamic acid and sodium azide under Bi(OTf)3 catalysis. Synthesis 45:507–510. https://doi.org/10.1055/s-0032-1318108
Abdollahi-Alibeik M, Moaddeli A (2015) Multi-component one-pot reaction of aldehyde, hydroxylamine and sodium azide catalyzed by Cu–MCM-41 nanoparticles: a novel method for the synthesis of 5-substituted 1H-tetrazole derivatives. New J Chem 39:2116–2122. https://doi.org/10.1039/C4NJ01042F
Khan KM, Fatima I, Saad SM, Taha M, Voelter W (2016) An efficient one-pot protocol for the conversion of benzaldehydes into tetrazole analogs. Tetrahedron Lett 57:523–524. https://doi.org/10.1016/j.tetlet.2015.12.067
Heravi MM, Fazeli A, Oskooie HA, Beheshtiha YS, Valizadeh H (2012) Click synthesis of 5-substituted 1H-tetrazoles from aldehydes, hydroxylamine, and [bmim]N3 via one-pot, three-component reaction. Synlett 23:2927–2930. https://doi.org/10.1055/s-0032-1317671
Esmaeilpour M, Sardarian AR, Firouzabadi H (2018) Dendrimer-encapsulated Cu(II) nanoparticles immobilized on superparamagnetic Fe3O4@SiO2 nanoparticles as a novel recyclable catalyst for N-arylation of nitrogen heterocycles and green synthesis of 5-substituted 1H-tetrazoles. Appl Organometal Chem 32:e4300. https://doi.org/10.1002/aoc.4300
Mitra B, Mukherjee S, Pariyar GC, Ghosh P (2018) One pot three-component synthesis of 5-substituted 1H-tetrazole from aldehyde. Tetrahedron Lett 14:1385–1389. https://doi.org/10.1016/j.tetlet.2018.02.067
Behrouz S (2017) Highly efficient three-component synthesis of 5-substituted-1H-tetrazoles from aldehydes, hydroxylamine, and tetrabutylammonium azide using doped nano-sized copper(I) oxide (Cu2O) on melamine–formaldehyde resin. J Saudi Chem Soc 21:220–228. https://doi.org/10.1002/anie.200701045
Patil UB, Kumthekar KR, Nagarkar JM (2012) A novel method for the synthesis of 5-substituted 1H-tetrazole from oxime and sodium azide. Tetrahedron Lett 53:3706–3709. https://doi.org/10.1016/j.tetlet.2012.04.093
Guggilapu SD, Prajapti SK, Nagarsenkar A, Gupta KK, Babu BN (2016) Indium(III) chloride catalyzed synthesis of 5-substituted 1H-tetrazoles from oximes and sodium azide. Synlett 27:1241–1244. https://doi.org/10.1055/s-0035-1561559
Arghan M, Koukabi N, Kolvari E (2018) Mizoroki-Heck and Suzuki-Miyaura reactions mediated by poly (2-acrylamido-2-methyl-1-propanesulfonic acid)-stabilized magnetically separable palladium catalyst. Appl Organometal Chem 32:e4346. https://doi.org/10.1002/aoc.4346
Koukabi N, Kolvari E, Zolfigol MA, Khazaei A, Shaghasemi BS, Fasahati B (2012) A magnetic particle-supported sulfonic acid catalyst: tuning catalytic activity between homogeneous and heterogeneous catalysis. Adv Synth Catal 354:2001–2008. https://doi.org/10.1002/adsc.201100352
Koukabi N, Kolvari E, Khazaei A, Zolfigol MA, Shirmardi-Shaghasemi B, Khavasi HR (2011) Hantzsch reaction on free nano-Fe2O3 catalyst: excellent reactivity combined with facile catalyst recovery and recyclability. Chem Comm 47:9230–9232. https://doi.org/10.1039/C1CC12693H
Kolvari E, Koukabi N, Hosseini MM (2015) Perlite: a cheap natural support for immobilization of sulfonic acid as a heterogeneous solid acid catalyst for the heterocyclic multicomponent reaction. J Mol Catal Chem 397:68–75. https://doi.org/10.1016/j.molcata.2014.10.026
Kolvari E, Koukabi N, Hosseini MM, Vahidian M, Ghobadi E (2016) Nano-ZrO2 sulfuric acid: a heterogeneous solid acid nano catalyst for Biginelli reaction under solvent free conditions. RSC Adv 6:7419–7425. https://doi.org/10.1039/C5RA19350H
Akbarzadeh P, Koukabi N, Kolvari E (2019) Three-component solvent-free synthesis of 5-substituted-1H-tetrazoles catalyzed by unmodified nanomagnetite with microwave irradiation or conventional heating. Res Chem Intermed 45:1009–1024. https://doi.org/10.1007/s11164-018-3657-9
Lotfi Z, Mousavi HZ, Sajjadi SM (2017) Magnetic carbon nanotubes modified with 1,4-diazabicyclo [2.2.2] octane are a viable sorbent for extraction of selective serotonin reuptake inhibitors. Microchim Acta 184:1427–1436. https://doi.org/10.1007/s00604-017-2150-2
Li J, Tang S, Lu L, Zeng HC (2007) Preparation of nanocomposites of metals, metal oxides, and carbon nanotubes via self-assembly. J Am Chem Soc 129:9401–9409. https://doi.org/10.1021/ja071122v
Sabaqian S, Nemati F, Nahzomi HT, Heravi MM (2018) Silver(I) dithiocarbamate on modified magnetic cellulose: synthesis, density functional theory study and application. Carbohydr Polym 184:221–230. https://doi.org/10.1016/j.carbpol.2017.12.045
Chidambaram S, Pari B, Kasi N, Muthusamy S (2016) ZnO/Ag heterostructures embedded in Fe3O4 nanoparticles for magnetically recoverable photocatalysis. J Alloys Compd 665:404–410. https://doi.org/10.1016/j.jallcom.2015.11.011
Acknowledgements
The authors gratefully acknowledge the Semnan University Research Council for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Akbarzadeh, P., Koukabi, N. & Kolvari, E. Anchoring of triethanolamine–Cu(II) complex on magnetic carbon nanotube as a promising recyclable catalyst for the synthesis of 5-substituted 1H-tetrazoles from aldehydes. Mol Divers 24, 319–333 (2020). https://doi.org/10.1007/s11030-019-09951-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-019-09951-6