Abstract
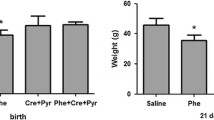
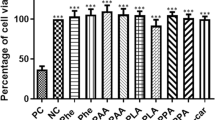
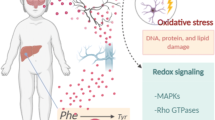
Phenylketonuria (PKU) is the most common inborn error of amino acid metabolism. Usually diagnosed within the first month of birth, it is essential that the patient strictly follow the dietary restriction of natural protein intake. Otherwise, PKU impacts the development of the brain severely and may result in microcephaly, epilepsy, motor deficits, intellectual disability, and psychiatric and behavioral disorders. The neuropathology associated with PKU includes defects of myelination, insufficient synthesis of monoamine neurotransmitters, amino acid imbalance across the blood-brain barrier, and involves intermediary metabolic pathways supporting energy homeostasis and antioxidant defenses in the brain. Considering that the production of reactive oxygen species (ROS) is inherent to energy metabolism, we investigated the association of creatine+pyruvate (Cr + Pyr), both energy substrates with antioxidants properties, as a possible treatment to mitigate oxidative stress and phosphotransfer network impairment elicited in the brain of young Wistar rats by chemically-induced PKU. We induced PKU through the administration of α-methyl-L-phenylalanine and phenylalanine for 7 days, with and without Cr + Pyr supplementation, until postpartum day 14. The cotreatment with Cr + Pyr administered concurrently with PKU induction prevented ROS formation and part of the alterations observed in antioxidants defenses and phosphotransfer network enzymes in the cerebral cortex, hippocampus, and cerebellum. If such prevention also occurs in PKU patients, supplementing the phenylalanine-restricted diet with antioxidants and energetic substrates might be beneficial to these patients.


Similar content being viewed by others
Abbreviations
- PKU:
-
Phenylketonuria
- IEM:
-
Inborn error of metabolism
- PAH:
-
Phenylalanine hydroxylase
- Phe:
-
Phenylalanine
- HPA:
-
Hyperphenylalaninemia
- ROS:
-
Reactive oxygen species
- Cr:
-
Creatine
- Pyr:
-
Pyruvate
- αMePhe:
-
α-methyl-L-phenylalanine
- DCFH:
-
2′,7′-dihydrodichlorofluorescein
- SH:
-
Sulfhydryl groups
- GSH:
-
Reduced glutathione
- CAT:
-
Catalase
- SOD:
-
Superoxide dismutase
- GPx:
-
Glutathione peroxidase
- CytCK:
-
Cytosolic creatine kinase
- MtCK:
-
Mitochondrial creatine kinase
- PCr:
-
Phosphocreatine
- PK:
-
Pyruvate kinase
- HK:
-
Hexokinase
- AK:
-
Adenylate kinase
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- OXPHOS:
-
Oxidative phosphorylation
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Aksenov MY, Markesbery WR (2001) Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer's disease. Neurosci Lett 302(2–3):141–145
Altman J (1969) Autoradiographic and histological studies of postnatal neurogenesis. 3. Dating the time of production and onset of differentiation of cerebellar microneurons in rats. J Comp Neurol 136(3):269–293
Anderson PJ, Leuzzi V (2010) White matter pathology in phenylketonuria. Mol Genet Metab 99(Suppl 1):S3–S9
Bandeira F, Lent R, Herculano-Houzel S (2009) Changing numbers of neuronal and non-neuronal cells underlie postnatal brain growth in the rat. Proc Natl Acad Sci U S A 106(33):14108–14113
Berti SL, Nasi GM, Garcia C, Castro FL, Nunes ML, Rojas DB, Moraes TB, Dutra-Filho CS, Wannmacher CM (2012) Pyruvate and creatine prevent oxidative stress and behavioral alterations caused by phenylalanine administration into hippocampus of rats. Metab Brain Dis 27(1):79–89
Bilder DA, Burton BK, Coon H, Leviton L, Ashworth J, Lundy BD, Vespa H, Bakian AV, Longo N (2013) Psychiatric symptoms in adults with phenylketonuria. Mol Genet Metab 108(3):155–160
Bilder DA, Noel JK, Baker ER, Irish W, Chen Y, Merilainen MJ, Prasad S, Winslow BJ (2016) Systematic review and meta-analysis of neuropsychiatric symptoms and executive functioning in adults with phenylketonuria. Dev Neuropsychol 41(4):245–260
Blau N (2016) Genetics of phenylketonuria: then and now. Hum Mutat 37(6):508–515
Bodner KE, Aldridge K, Moffitt AJ, Peck D, White DA, Christ SE (2012) A volumetric study of basal ganglia structures in individuals with early-treated phenylketonuria. Mol Genet Metab 107(3):302–307
Bortoluzzi VT, de Franceschi ID, Rieger E, Wannmacher CM (2014) Co-administration of creatine plus pyruvate prevents the effects of phenylalanine administration to female rats during pregnancy and lactation on enzymes activity of energy metabolism in cerebral cortex and hippocampus of the offspring. Neurochem Res 39(8):1594–1602
Browne RW, Armstrong D (1998) Reduced glutathione and glutathione disulfide. Methods Mol Biol 108:347–352
Cadenas E, Davies KJ (2000) Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 29(3–4):222–230
Calabrese V, Scapagnini G, Ravagna A, Fariello RG, Giuffrida Stella AM, Abraham NG (2002) Regional distribution of heme oxygenase, HSP70, and glutathione in brain: relevance for endogenous oxidant/antioxidant balance and stress tolerance. J Neurosci Res 68(1):65–75
Campese VM, Sindhu RK, Ye S, Bai Y, Vaziri ND, Jabbari B (2007) Regional expression of NO synthase, NAD(P)H oxidase and superoxide dismutase in the rat brain. Brain Res 1134(1):27–32
Cobley JN, Fiorello ML, Bailey DM (2018) 13 reasons why the brain is susceptible to oxidative stress. Redox Biol 15:490–503
Costabeber E, Kessler A, Severo Dutra-Filho C, de Souza Wyse AT, Wajner M, Wannmacher CM (2003) Hyperphenylalaninemia reduces creatine kinase activity in the cerebral cortex of rats. Int J Dev Neurosci 21(2):111–116
Dimer NW, Ferreira BK, Agostini JF, Gomes ML, Kist LW, Malgarin F, Carvalho-Silva M, Gomes LM, Rebelo J, Frederico MJS, Silva F, Rico EP, Bogo MR, Streck EL, Ferreira GC, Schuck PF (2018) Brain bioenergetics in rats with acute hyperphenylalaninemia. Neurochem Int 117:188–203
Dos Reis EA, Rieger E, de Souza SS, Rasia-Filho AA, Wannmacher CM (2013) Effects of a co-treatment with pyruvate and creatine on dendritic spines in rat hippocampus and posterodorsal medial amygdala in a phenylketonuria animal model. Metab Brain Dis 28(3):509–517
Dzeja PP, Terzic A (2003) Phosphotransfer networks and cellular energetics. J Exp Biol 206(Pt 12):2039–2047
Dzeja P, Terzic A (2009) Adenylate kinase and AMP signaling networks: metabolic monitoring, signal communication and body energy sensing. Int J Mol Sci 10(4):1729–1772
Dzeja P, Kalvenas A, Toleikis A, Praskevicius A (1985) The effect of adenylate kinase activity on the rate and efficiency of energy transport from mitochondria to hexokinase. Biochem Int 10(2):259–265
Dzeja PP, Hoyer K, Tian R, Zhang S, Nemutlu E, Spindler M, Ingwall JS (2011) Rearrangement of energetic and substrate utilization networks compensate for chronic myocardial creatine kinase deficiency. J Physiol 589(Pt 21):5193–5211
Feksa LR, Cornelio AR, Rech VC, Dutra-Filho CS, Wyse AT, Wajner M, Wannmacher CM (2002) Alanine prevents the reduction of pyruvate kinase activity in brain cortex of rats subjected to chemically induced hyperphenylalaninemia. Neurochem Res 27(9):947–952
Feksa LR, Cornelio AR, Dutra-Filho CS, de Souza Wyse AT, Wajner M, Wannmacher CM (2003) Characterization of the inhibition of pyruvate kinase caused by phenylalanine and phenylpyruvate in rat brain cortex. Brain Res 968(2):199–205
Ficicioglu C, Dubroff JG, Thomas N, Gallagher PR, Burfield J, Hussa C, Randall R, Zhuang H (2013) A pilot study of Fluorodeoxyglucose positron emission tomography findings in patients with phenylketonuria before and during Sapropterin supplementation. J Clin Neurol 9(3):151–156
Gal S, Zheng H, Fridkin M, Youdim MB (2005) Novel multifunctional neuroprotective iron chelator-monoamine oxidase inhibitor drugs for neurodegenerative diseases. In vivo selective brain monoamine oxidase inhibition and prevention of MPTP-induced striatal dopamine depletion. J Neurochem 95(1):79–88
Gonzalez MJ, Gutierrez AP, Gassio R, Fuste ME, Vilaseca MA, Campistol J (2011) Neurological complications and behavioral problems in patients with phenylketonuria in a follow-up unit. Mol Genet Metab 104(Suppl):S73–S79
de Groot MJ, Hoeksma M, Reijngoud DJ, de Valk HW, Paans AM, Sauer PJ, van Spronsen FJ (2013) Phenylketonuria: reduced tyrosine brain influx relates to reduced cerebral protein synthesis. Orphanet J Rare Dis 8:133
Halliwell B (1992) Reactive oxygen species and the central nervous system. J Neurochem 59(5):1609–1623
Hildebrandt T, Knuesting J, Berndt C, Morgan B, Scheibe R (2015) Cytosolic thiol switches regulating basic cellular functions: GAPDH as an information hub? Biol Chem 396(5):523–537
Horster F, Schwab MA, Sauer SW, Pietz J, Hoffmann GF, Okun JG, Kolker S, Kins S (2006) Phenylalanine reduces synaptic density in mixed cortical cultures from mice. Pediatr Res 59(4 Pt 1):544–548
Hughes BP (1962) A method for the estimation of serum creatine kinase and its use in comparing creatine kinase and aldolase activity in normal and pathological sera. Clin Chim Acta 7:597–603
Kayaalp E, Treacy E, Waters PJ, Byck S, Nowacki P, Scriver CR (1997) Human phenylalanine hydroxylase mutations and hyperphenylalaninemia phenotypes: a metanalysis of genotype-phenotype correlations. Am J Hum Genet 61(6):1309–1317
Kienzle Hagen ME, Pederzolli CD, Sgaravatti AM, Bridi R, Wajner M, Wannmacher CM, Wyse AT, Dutra-Filho CS (2002) Experimental hyperphenylalaninemia provokes oxidative stress in rat brain. Biochim Biophys Acta 1586(3):344–352
Kladna A, Marchlewicz M, Piechowska T, Kruk I, Aboul-Enein HY (2015) Reactivity of pyruvic acid and its derivatives towards reactive oxygen species. Luminescence 30(7):1153–1158
Korshunov SS, Skulachev VP, Starkov AA (1997) High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett 416(1):15–18
Lawler JM, Barnes WS, Wu G, Song W, Demaree S (2002) Direct antioxidant properties of creatine. Biochem Biophys Res Commun 290(1):47–52
LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5(2):227–231
Leong SF, Lai JC, Lim L, Clark JB (1981) Energy-metabolizing enzymes in brain regions of adult and aging rats. J Neurochem 37(6):1548–1556
Long LH, Halliwell B (2009) Artefacts in cell culture: pyruvate as a scavenger of hydrogen peroxide generated by ascorbate or epigallocatechin gallate in cell culture media. Biochem Biophys Res Commun 388(4):700–704
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
MacDonald A, Gokmen-Ozel H, van Rijn M, Burgard P (2010) The reality of dietary compliance in the management of phenylketonuria. J Inherit Metab Dis 33(6):665–670
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47(3):469–474
Martynyuk AE, Glushakov AV, Sumners C, Laipis PJ, Dennis DM, Seubert CN (2005) Impaired glutamatergic synaptic transmission in the PKU brain. Mol Genet Metab 86(Suppl 1):S34–S42
Mazzola JL, Sirover MA (2005) Aging of human glyceraldehyde-3-phosphate dehydrogenase is dependent on its subcellular localization. Biochim Biophys Acta 1722(2):168–174
Meyer LE, Machado LB, Santiago AP, da Silva WS, De Felice FG, Holub O, Oliveira MF, Galina A (2006) Mitochondrial creatine kinase activity prevents reactive oxygen species generation: antioxidant role of mitochondrial kinase-dependent ADP re-cycling activity. J Biol Chem 281(49):37361–37371
Miller AL, Hawkins RA, Veech RL (1973) Phenylketonuria: phenylalanine inhibits brain pyruvate kinase in vivo. Science 179(4076):904–906
Moraes TB, Jacques CE, Rosa AP, Dalazen GR, Terra M, Coelho JG, Dutra-Filho CS (2013) Role of catalase and superoxide dismutase activities on oxidative stress in the brain of a phenylketonuria animal model and the effect of lipoic acid. Cell Mol Neurobiol 33(2):253–260
Moro N, Ghavim SS, Harris NG, Hovda DA, Sutton RL (2016) Pyruvate treatment attenuates cerebral metabolic depression and neuronal loss after experimental traumatic brain injury. Brain Res 1642:270–277
Pearsen KD, Gean-Marton AD, Levy HL, Davis KR (1990) Phenylketonuria: MR imaging of the brain with clinical correlation. Radiology 177(2):437–440
Perez-Duenas B, Pujol J, Soriano-Mas C, Ortiz H, Artuch R, Vilaseca MA, Campistol J (2006) Global and regional volume changes in the brains of patients with phenylketonuria. Neurology 66(7):1074–1078
Pfaendner NH, Reuner G, Pietz J, Jost G, Rating D, Magnotta VA, Mohr A, Kress B, Sartor K, Hahnel S (2005) MR imaging-based volumetry in patients with early-treated phenylketonuria. AJNR Am J Neuroradiol 26(7):1681–1685
Pietz J, Rupp A, Ebinger F, Rating D, Mayatepek E, Boesch C, Kreis R (2003) Cerebral energy metabolism in phenylketonuria: findings by quantitative in vivo 31P MR spectroscopy. Pediatr Res 53(4):654–662
Preissler T, Bristot IJ, Costa BM, Fernandes EK, Rieger E, Bortoluzzi VT, de Franceschi ID, Dutra-Filho CS, Moreira JC, Wannmacher CM (2016) Phenylalanine induces oxidative stress and decreases the viability of rat astrocytes: possible relevance for the pathophysiology of neurodegeneration in phenylketonuria. Metab Brain Dis 31(3):529–537
Rae CD, Broer S (2015) Creatine as a booster for human brain function. How might it work? Neurochem Int 89:249–259
Rausell D, García-Blanco A, Correcher P, Vitoria I, Vento M, Cháfer-Pericás C (2019) Newly validated biomarkers of brain damage may shed light into the role of oxidative stress in the pathophysiology of neurocognitive impairment in dietary restricted phenylketonuria patients. Pediatr Res 85(2):242–250
Rech VC, Feksa LR, Dutra-Filho CS, Wyse AT, Wajner M, Wannmacher CM (2002) Inhibition of the mitochondrial respiratory chain by phenylalanine in rat cerebral cortex. Neurochem Res 27(5):353–357
Reichmann D, Voth W, Jakob U (2018) Maintaining a healthy proteome during oxidative stress. Mol Cell 69(2):203–213
Ribas GS, Sitta A, Wajner M, Vargas CR (2011) Oxidative stress in phenylketonuria: what is the evidence? Cell Mol Neurobiol 31(5):653–662
Ryu JK, Choi HB, McLarnon JG (2006) Combined minocycline plus pyruvate treatment enhances effects of each agent to inhibit inflammation, oxidative damage, and neuronal loss in an excitotoxic animal model of Huntington's disease. Neuroscience 141(4):1835–1848
Salminen LE, Paul RH (2014) Oxidative stress and genetic markers of suboptimal antioxidant defense in the aging brain: a theoretical review. Rev Neurosci 25(6):805–819
Sanayama Y, Nagasaka H, Takayanagi M, Ohura T, Sakamoto O, Ito T, Ishige-Wada M, Usui H, Yoshino M, Ohtake A, Yorifuji T, Tsukahara H, Hirayama S, Miida T, Fukui M, Okano Y (2011) Experimental evidence that phenylalanine is strongly associated to oxidative stress in adolescents and adults with phenylketonuria. Mol Genet Metab 103(3):220–225
Schlattner U, Klaus A, Ramirez Rios S, Guzun R, Kay L, Tokarska-Schlattner M (2016) Cellular compartmentation of energy metabolism: creatine kinase microcompartments and recruitment of B-type creatine kinase to specific subcellular sites. Amino Acids 48(8):1751–1774
Schuck PF, Malgarin F, Cararo JH, Cardoso F, Streck EL, Ferreira GC (2015) Phenylketonuria pathophysiology: on the role of metabolic alterations. Aging Dis 6(5):390–399
Scriver CR (1995) The metabolic and molecular bases of inherited disease. McGraw-Hill, Health Professions Division, New York
da Silva WS, Gomez-Puyou A, de Gomez-Puyou MT, Moreno-Sanchez R, De Felice FG, de Meis L, Oliveira MF, Galina A (2004) Mitochondrial bound hexokinase activity as a preventive antioxidant defense: steady-state ADP formation as a regulatory mechanism of membrane potential and reactive oxygen species generation in mitochondria. J Biol Chem 279(38):39846–39855
Sirtori LR, Dutra-Filho CS, Fitarelli D, Sitta A, Haeser A, Barschak AG, Wajner M, Coelho DM, Llesuy S, Bello-Klein A, Giugliani R, Deon M, Vargas CR (2005) Oxidative stress in patients with phenylketonuria. Biochim Biophys Acta 1740(1):68–73
Smith, R. N., A. S. Agharkar and E. B. Gonzales (2014). "A review of creatine supplementation in age-related diseases: more than a supplement for athletes." F1000Res 3: 222
Stepien KM, Heaton R, Rankin S, Murphy A, Bentley J, Sexton D, Hargreaves IP (2017) Evidence of oxidative stress and secondary mitochondrial dysfunction in metabolic and non-metabolic disorders. J Clin Med 6(7)
Stockler S, Holzbach U, Hanefeld F, Marquardt I, Helms G, Requart M, Hanicke W, Frahm J (1994) Creatine deficiency in the brain: a new, treatable inborn error of metabolism. Pediatr Res 36(3):409–413
Suh SW, Aoyama K, Matsumori Y, Liu J, Swanson RA (2005) Pyruvate administered after severe hypoglycemia reduces neuronal death and cognitive impairment. Diabetes 54(5):1452–1458
Surtees R, Blau N (2000) The neurochemistry of phenylketonuria. Eur J Pediatr 159(Suppl 2):S109–S113
Veyrat-Durebex C, Debeissat C, Blasco H, Patin F, Henique H, Emond P, Antar C, Gissot V, Herault O, Maillot F (2017) Hyperphenylalaninemia correlated with global decrease of antioxidant genes expression in White blood cells of adult patients with phenylketonuria. JIMD Rep 37:73–83
Wasserstein MP, Snyderman SE, Sansaricq C, Buchsbaum MS (2006) Cerebral glucose metabolism in adults with early treated classic phenylketonuria. Mol Genet Metab 87(3):272–277
Weber G (1969) Inhibition of human brain pyruvate kinase and hexokinase by phenylalanine and phenylpyruvate: possible relevance to phenylketonuric brain damage. Proc Natl Acad Sci U S A 63(4):1365–1369
van Wegberg AMJ, MacDonald A, Ahring K, Belanger-Quintana A, Blau N, Bosch AM, Burlina A, Campistol J, Feillet F, Gizewska M, Huijbregts SC, Kearney S, Leuzzi V, Maillot F, Muntau AC, van Rijn M, Trefz F, Walter JH, van Spronsen FJ (2017) The complete European guidelines on phenylketonuria: diagnosis and treatment. Orphanet J Rare Dis 12(1):162
Wendel A (1981) Glutathione peroxidase. Methods Enzymol 77:325–333
Yu YM, Kim JB, Lee KW, Kim SY, Han PL, Lee JK (2005) Inhibition of the cerebral ischemic injury by ethyl pyruvate with a wide therapeutic window. Stroke 36(10):2238–2243
Yuan M, McNae IW, Chen Y, Blackburn EA, Wear MA, Michels PAM, Fothergill-Gilmore LA, Hupp T, Walkinshaw MD (2018) An allostatic mechanism for M2 pyruvate kinase as an amino-acid sensor. Biochem J 475(10):1821–1837
Zhang H, Gu XF (2005) A study of gene expression profiles of cultured embryonic rat neurons induced by phenylalanine. Metab Brain Dis 20(1):61–72
Zorova LD, Popkov VA, Plotnikov EY, Silachev DN, Pevzner IB, Jankauskas SS, Babenko VA, Zorov SD, Balakireva AV, Juhaszova M, Sollott SJ, Zorov DB (2018) Mitochondrial membrane potential. Anal Biochem 552:50–59
Acknowledgements
The authors express their gratitude to the Department of Biochemistry, and the Basic Health Sciences Institute of the Federal University of Rio Grande do Sul, especially to the people responsible for animal care. We also thank the funding agencies that supported the present study – Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Financiadora de Estudos e Projetos (FINEP) Rede Instituto Brasileiro de Neurociência.
Funding
This study was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Financiadora de Estudos e Projetos (FINEP) Rede Instituto Brasileiro de Neurociência.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflict of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 323 kb)
Rights and permissions
About this article
Cite this article
Bortoluzzi, V.T., Brust, L., Preissler, T. et al. Creatine plus pyruvate supplementation prevents oxidative stress and phosphotransfer network disturbances in the brain of rats subjected to chemically-induced phenylketonuria. Metab Brain Dis 34, 1649–1660 (2019). https://doi.org/10.1007/s11011-019-00472-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-019-00472-7