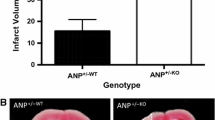
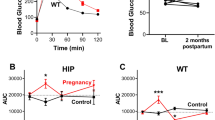
Abstract
Pregnancy evokes many challenges on the maternal cardiovascular system that may unmask predispositions for future disease. This is particularly evident for women who develop pregnancy-related disorders, for example, pre-eclampsia and gestational diabetes or hypertension. Such pregnancy-related syndromes increase the risk for cardiovascular disease (CVD) postpartum. As a result, pregnancy has been termed as a cardiovascular stress test and an indicator or marker to predict the development of CVD later in life. In addition, pregnancy-related disorders impact the development of offspring also placing them at a higher risk for disease. Utilizing pregnancy as a physiological stressor, the current investigation sought to determine whether the cardiovascular system of offspring exposed to gestational hypertension in utero would respond adversely to the stress of pregnancy. Heterozygous atrial natriuretic peptide gene-disrupted (ANP+/−) offspring were generated by either crossing male wildtype ANP+/+ with female knockout ANP−/− to produce ANP+/−KO mice or crossing female wildtype ANP+/+ with male knockout ANP−/− to produce ANP+/−WT mice. To study the cardiovascular stress induced by pregnancy, female ANP+/−WT and ANP+/−KO mice were mated with male wildtype ANP+/+ mice to initiate pregnancy. Cardiac size and molecular expression of the renin-angiotensin (RAS) and natriuretic peptide systems (NPS) were compared between offspring groups. Our data demonstrate that gestational hypertension and lack of maternal ANP did not significantly impact the progression and regression of pregnancy-induced cardiac hypertrophy over gestation and postpartum in ANP+/− offspring. Additionally, the molecular cardiac expression of the RAS and NPS did not differ between offspring groups. Future investigation should assess potential differences in cardiac function and the impact of fetal-programming on offspring cardiovascular adaptations during pregnancy in more severe models of pregnancy-related hypertensive syndrome such as angiotensin II or isoproterenol infusion.





Similar content being viewed by others
References
Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME (1989) Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. Br Med J 298:564–567
Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ (1989) Weight in infancy and death from ischaemic heart disease. Lancet 2(8663):577–580
Ma RC, Tutino GE, Lillycrop KA, Hanson MA, Tam WH (2015) Maternal diabetes, gestational diabetes and the role of epigenetics in their long term effects on offspring. Prog Biophys Mol Biol 118(1–2):55–68
Petropoulos S, Guillemin C, Ergaz Z, Dimov S, Suderman M, Weinstein-Fudim L, Ornoy A, Szyf M (2015) Gestational diabetes alters offspring DNA methylation profiles in human and rat: identification of key pathways involved in endocrine system disorders, insulin signaling, diabetes signaling and ILK signaling. Endocrinology 156(6):2222–2238
Wilson BJ, Watson MS, Prescott GJ, Sunderland S, Campbell DM, Hannaford P, Smith WC (2003) Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. Br Med J 326:845–849
Anderson CM (2007) Preeclampsia: exposing future cardiovascular risk in mothers and their children. J Obstet Gynecol Neonatal Nurs 36(1):3–8
Kajantie E, Eriksson JG, Osmond C, Thornburg K, Barker DJP (2009) Pre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort study. Stroke 40(4):1176–1180
Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, Adwani S, Wilkinson AR, McCormick K, Sargent I, Redman C, Leeson P (2012) Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics 129(6):e1552–e1561
Wu G, Bazer FW, Cudd TA, Meininger CJ, Spencer TE (2004) Maternal nutrition and fetal development. J Nutr 134(9):2169–2172
Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S (2009) Fetuses of obese mothers develop insulin resistance in utero. Diabet Care 32(6):1076–1080
Molkentin JD (2003) A friend within the heart: natriuretic peptide receptor signaling. J Clin Invest 111(9):1275–1277
Potter LR, Abbey-Hosch S, Dickey DM (2006) Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev 27(1):47–72
John SW, Krege JH, Oliver PM, Hagaman JR, Hodgin JB, Pang SC, Flynn TG, Smithies O (1995) Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science 267:679–681
Feng JA, Perry G, Mori T, Hayashi T, Oparil S, Chen Y-F (2003) Pressure-independent enhancement of cardiac hypertrophy in atrial natriuretic peptide-deficient mice. Clin Exp Pharmacol Physiol 30:343–349
Armstrong DWJ, Tse MY, O’Tierney-Ginn PF, Wong PG, Ventura NM, Janzen-Pang JJ, Matangi MF, Johri AM, Croy BA, Adams MA, Pang SC (2013) Gestational hypertension in atrial natriuretic peptide knockout mice and the developmental origins of salt-sensitivity and cardiac hypertrophy. Regul Pept 186:108–115
Armstrong DWJ, Tse MY, Wong PG, Ventura NM, Meens JA, Johri AM, Matangi MF, Pang SC (2014) Gestational hypertension and the developmental origins of cardiac hypertrophy and diastolic dysfunction. Mol Cell Biochem 391:201–209
Ventura NM, Jin AY, Tse MY, Peterson NT, Andrew RD, Mewburn JD, Pang SC (2015) Maternal hypertension programs increased cerebral tissue damage following stroke in adult offspring. Mol Cell Biochem 408:223–233
Melchiorre K, Sharma R, Thilaganathan B (2012) Cardiac structure and function in normal pregnancy. Curr Opin Obstet Gynecol 24:413–421
Sanghavi M, Rutherford JD (2014) Cardiovascular physiology of pregnancy. Circulation 130:1003–1008
Williams D (2003) Pregnancy: a stress test for life. Curr Opin Obstet Gynecol 15:465–471
Smith GN, Walker MC, Liu A, Wen SW, Swansburg M, Ramshaw H, White RR, Roddy M, Hladunewich M (2009) A history of preeclampsia identifies women who have underlying cardiovascular risk factors. Am J Obstet Gynecol 200(1):58.e1–58.e8
Ventura NM, Peterson NT, Tse MY, Andrew RD, Pang SC, Jin AY (2015) Molecular adaptations in vasoactive systems during acute stroke in salt-induced hypertension. Mol Cell Biochem 399:39–47
Hytten F (1985) Blood volume changes in normal pregnancy. Clin Haematol 14(3):601–612
Geva T, Mauer MB, Striker L, Kirshon B, Pivarnik JM (1997) Effects of physiologic load of pregnancy on left ventricular contractility and remodeling. Am Heart J 133:53–59
Li J, Umar S, Amjedi M, Iorga A, Sharma S, Nadadur RD, Regitz-Zagrosek V, Eghbali M (2012) New frontiers in heart hypertrophy during pregnancy. Am J Cardiovasc Dis 2:192–207
Estensen ME, Beitnes JO, Grindheim G, Aaberge L, Smiseth OA, Henriksen T, Aakhus S (2013) Altered maternal left ventricular contractility and function during normal pregnancy. Ultrasound Obstet Gynecol 41(6):659–666
McMullen JR, Jennings GL (2007) Differences between pathological and physiological cardiac hypertrophy: novel therapeutic strategies to treat heart failure. Clin Exp Pharmacol Physiol 34(4):255–262
Umar S, Nadadur R, Iorga A, Amjedi M, Matori H, Eghbali M (2012) Cardiac structural and hemodynamic changes associated with physiological heart hypertrophy of pregnancy are reversed postpartum. J Appl Physiol 113:1253–1259
Clapp JF III, Capeless E (1997) Cardiovascular function before, during, and after the first and subsequent pregnancies. Am J Cardiol 80:1469–1473
Irani RA, Xia Y (2011) Renin angiotensin signaling in normal pregnancy and preeclampsia. Semin Nephrol 31(1):47–58
Langer B, Grima M, Coquard C, Bader AM, Schlaeder G, Imbs JL (1998) Plasma active renin, angiotensin I, and angiotensin II during pregnancy and in preeclampsia. Obstet Gynecol 91:196–202
Merrill DC, Karoly M, Chen K, Ferrario CM, Brosnihan KB (2002) Angiotensin-(1–7) in normal and preeclamptic pregnancy. Endocrine 18:239–245
Brosnihan KB, Neves LAA, Anton L, Joyner J, Valdes G, Merrill DC (2004) Enhanced expression of Ang-(1–7) during pregnancy. Braz J Med Biol Res 37:1255–1262
Peach MJ (1977) Renin-angiotensin system: biochemistry and mechanisms of action. Physiol Rev 57:313–370
Ventura NM, Li TY, Tse MY, Andrew RD, Tayade C, Jin AY, Pang SC (2015) Onset and regression of pregnancy-induced cardiac alterations in gestationally hypertensive mice: the role of the natriuretic peptide system. Biol Reprod 93:142–149
Kuroski de Bold ML (1998) Atrial natriuretic factor and brain natriuretic peptide gene expression in the spontaneous hypertensive rat during postnatal development. Am J Hypertens 11(8 Pt 1):1006–1018
Nagaya N, Nishikimi T, Goto Y, Miyao Y, Kobayashi Y, Morii I, Daikoku S, Matsumoto T, Miyazaki S, Matsuoka H, Takishita S, Kaqawa K, Matsuo H, Nonoqi H (1998) Plasma brain natriuretic peptide is a biochemical marker for the prediction of progressive ventricular remodeling after acute myocardial infarction. Am Heart J 135:21–28
Wang D, Oparil S, Feng JA, Li P, Perry G, Chen LB, Dai M, John SWM, Chen Y-F (2003) Effects of pressure overload on extracellular matrix expression in the heart of the atrial natriuretic peptide–null mouse. Hypertension 42:88–95
Bernardo BC, Weeks KL, Pretorius L, McMullen JR (2010) Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther 128:191–227
Thomsen JK, Storm TL, Thamsborg G, de Nully M, Bødker B, Skouby SO (1988) Increased concentration of circulating atrial natriuretic peptide during normal pregnancy. Eur J Obstet Gynecol Reprod Biol 27:197–201
Kristensen CG, Nakagawa Y, Coe FL, Lindheimer MD (1986) Effect of atrial natriuretic factor in rat pregnancy. Am J Physiol 250(4 pt2):R589–R594
Masilamani S, Castro L, Baylis C (1994) Pregnant rats are refractory to the natriuretic actions of atrial natriuretic peptide. Am J Physiol 267(6 Pt 2):R1611–R1616
Sala C, Campise M, Ambroso G, Motta T, Zanchetti A, Morganti A (1995) Atrial natriuretic peptide and hemodynamic changes during normal human pregnancy. Hypertension 25:631–636
Eghbali M, Deva R, Alioua A, Minosyan TY, Ruan H, Wang Y, Toro L, Stefani E (2005) Molecular and functional signature of heart hypertrophy during pregnancy. Circ Res 96:1208–1216
Thomsen JK, Fogh-Andersen N, Jaszczak P, Glese J (1993) Atrial natriuretic peptide (ANP) decrease during normal pregnancy as related to hemodynamic changes and volume regulation. Acta Obstet Gynecol Scand 72(2):103–110
Poulsen H, Sjöberg N-O, Stjernquist M, Zia E (1994) Atrial natriuretic peptide antagonizes the contractile effect of angiotensin II in the human uterine artery. Hum Reprod 9:1939–1943
Yoshimura T, Yoshimura M, Yasue H, Ito M, Okamura H, Mukoyama M, Nakao K (1994) Plasma concentration of atrial natriuretic peptide and brain natriuretic peptide during normal human pregnancy and the postpartum period. J Endocrinol 140:393–397
Funding
Heart and Stroke Foundation of Canada provided funding to SCP (Grant #: T-6140) and to RDA (Grant #: G-14-0006260). N.M.V. was the recipient of the R.S. McLaughlin Fellowship, Queen’s University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Ventura, N.M., Li, T.Y., Tse, M.Y. et al. Developmental origins of pregnancy-induced cardiac changes: establishment of a novel model using the atrial natriuretic peptide gene-disrupted mice. Mol Cell Biochem 449, 227–236 (2018). https://doi.org/10.1007/s11010-018-3359-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-018-3359-z