Abstract
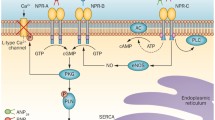
Studied for nearly 30 years for its ability to control many parameters, such as vascular smooth muscle cell relaxation, heart fibrosis, and kidney function, the natriuretic peptide (NP) system is now considered to be a key element in several other major metabolic pathways. After stimulation by NPs, natriuretic peptide receptors (NPR) convert GTP to the second messenger cGMP. In addition to its vasodilatory effects and natriuretic and diuretic functions, cGMP has been positively associated with fat cell function, apoptosis, and NPR expression/activity modulation. The NP system is also closely linked to metabolic syndrome (MetS) progression and obesity control. A new era is now on its way targeting the NP system to not only treat high blood pressure, but to also assist in the fight against the obesity pandemic. Here, we summarize recent data on the role of NPs in hypertension and MetS.



Similar content being viewed by others
References
de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H (1981) A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci 28:89–94
Anand-Srivastava MB, Srivastava AK, Cantin M (1987) Pertussis toxin attenuates atrial natriuretic factor-mediated inhibition of adenylate cyclase. Involvement of inhibitory guanine nucleotide regulatory protein. J Biol Chem 262:4931–4934
Anand-Srivastava MB, Trachte GJ (1993) Atrial natriuretic factor receptors and signal transduction mechanisms. Pharmacol Rev 45:455–497
Beavo JA, Brunton LL (2002) Cyclic nucleotide research—still expanding after half a century. Nat Rev Mol Cell Biol 3:710–718. doi:10.1038/nrm911
Bennett BD, Bennett GL, Vitangcol RV, Jewett JR, Burnier J, Henzel W, Lowe DG (1991) Extracellular domain-IgG fusion proteins for three human natriuretic peptide receptors. Hormone pharmacology and application to solid phase screening of synthetic peptide antisera. J Biol Chem 266:23060–23067
Chen HH, Burnett JC Jr (1998) C-type natriuretic peptide: the endothelial component of the natriuretic peptide system. J Cardiovasc Pharmacol 32(Suppl 3):S22–S28
Edwards BS, Zimmerman RS, Schwab TR, Heublein DM, Burnett JC Jr (1988) Atrial stretch, not pressure, is the principal determinant controlling the acute release of atrial natriuretic factor. Circ Res 62:191–195
Garbers DL (1991) Guanylyl cyclase-linked receptors. Pharmacol Ther 50:337–345. doi:10.1016/0163-7258(91)90049-R
Garbers DL, Lowe DG (1994) Guanylyl cyclase receptors. J Biol Chem 269:30741–30744
Grepin C, Dagnino L, Robitaille L, Haberstroh L, Antakly T, Nemer M (1994) A hormone-encoding gene identifies a pathway for cardiac but not skeletal muscle gene transcription. Mol Cell Biol 14:3115–3129
Hamet P, Tremblay J, Pang SC, Garcia R, Thibault G, Gutkowska J, Cantin M, Genest J (1984) Effect of native and synthetic atrial natriuretic factor on cyclic GMP. Biochem Biophys Res Commun 123:515–527. doi:10.1016/0006-291X(84)90260-2
Maack T, Suzuki M, Almeida FA, Nussenzveig D, Scarborough RM, McEnroe GA, Lewicki JA (1987) Physiological role of silent receptors of atrial natriuretic factor. Science 238:675–678. doi:10.1126/science.2823385
Porter JG, Arfsten A, Fuller F, Miller JA, Gregory LC, Lewicki JA (1990) Isolation and functional expression of the human atrial natriuretic peptide clearance receptor cDNA. Biochem Biophys Res Commun 171:796–803. doi:10.1016/0006-291X(90)91216-F
Potter LR, Hunter T (2001) Guanylyl cyclase-linked natriuretic peptide receptors: structure and regulation. J Biol Chem 276:6057–6060. doi:10.1074/jbc.R000033200
Sudoh T, Kangawa K, Minamino N, Matsuo H (1988) A new natriuretic peptide in porcine brain. Nature 332:78–81. doi:10.1038/332078a0
Sudoh T, Minamino N, Kangawa K, Matsuo H (1990) C-type natriuretic peptide (CNP): a new member of natriuretic peptide family identified in porcine brain. Biochem Biophys Res Commun 168:863–870. doi:10.1016/0006-291X(90)92401-K
Takei Y (2001) Does the natriuretic peptide system exist throughout the animal and plant kingdom? Comp Biochem Physiol B 129:559–573. doi:10.1016/S1096-4959(01)00366-9
Thuerauf DJ, Hanford DS, Glembotski CC (1994) Regulation of rat brain natriuretic peptide transcription. A potential role for GATA-related transcription factors in myocardial cell gene expression. J Biol Chem 269:17772–17775
Tremblay J, Gerzer R, Pang SC, Cantin M, Genest J, Hamet P (1986) ANF stimulation of detergent-dispersed particulate guanylate cyclase from bovine adrenal cortex. FEBS Lett 194:210–214. doi:10.1016/0014-5793(86)80086-2
Yan W, Wu F, Morser J, Wu Q (2000) Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci USA 97:8525–8529
Kumar P, Arise KK, Pandey KN (2006) Transcriptional regulation of guanylyl cyclase/natriuretic peptide receptor-A gene. Peptides 27:1762–1769. doi:10.1016/j.peptides.2006.01.004
Garg R, Pandey KN (2005) Regulation of guanylyl cyclase/natriuretic peptide receptor-A gene expression. Peptides 26:1009–1023. doi:10.1016/j.peptides.2004.09.022
Tremblay J, Hum D, Sanchez R, Dumas P, Pravenec M, Krenova D, Kren V, Kunes J, Pausova Z, Gossard F, Hamet P (2003) TA repeat variation, Npr1 expression, and blood pressure: impact of the Ace locus. Hypertension 41:16–24. doi:10.1161/01.HYP.0000042664.75193.1B
Tremblay J, Huot C, Willenbrock RC, Bayard F, Gossard F, Fujio N, Koch C, Kuchel O, Debinski W, Hamet P (1993) Increased cyclic guanosine monophosphate production and overexpression of atrial natriuretic peptide A-receptor mRNA in spontaneously hypertensive rats. J Clin Invest 92:2499–2508. doi:10.1172/JCI116858
Cao L, Wu J, Gardner DG (1995) Atrial natriuretic peptide suppresses the transcription of its guanylyl cyclase-linked receptor. J Biol Chem 270:24891–24897. doi:10.1074/jbc.270.42.24891
Hum D, Besnard S, Sanchez R, Devost D, Gossard F, Hamet P, Tremblay J (2004) Characterization of a cGMP-response element in the guanylyl cyclase/natriuretic peptide receptor A gene promoter. Hypertension 43:1270–1278. doi:10.1161/01.HYP.0000126920.93207.53
Potter LR, Garbers DL (1994) Protein kinase C-dependent desensitization of the atrial natriuretic peptide receptor is mediated by dephosphorylation. J Biol Chem 269:14636–14642
Potter LR, Hunter T (2000) Activation of protein kinase C stimulates the dephosphorylation of natriuretic peptide receptor-B at a single serine residue: a possible mechanism of heterologous desensitization. J Biol Chem 275:31099–31106
Abbey SE, Potter LR (2002) Vasopressin-dependent inhibition of the C-type natriuretic peptide receptor, NPR-B/GC-B, requires elevated intracellular calcium concentrations. J Biol Chem 277:42423–42430. doi:10.1074/jbc.M206686200
Sun JZ, Oparil S, Lucchesi P, Thompson JA, Chen YF (2001) Tyrosine kinase receptor activation inhibits NPR-C in lung arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 281:L155–L163
Kishimoto I, Yoshimasa T, Suga S, Ogawa Y, Komatsu Y, Nakagawa O, Itoh H, Nakao K (1994) Natriuretic peptide clearance receptor is transcriptionally down-regulated by beta 2-adrenergic stimulation in vascular smooth muscle cells. J Biol Chem 269:28300–28308
Sun JZ, Chen SJ, Majid-Hasan E, Oparil S, Chen YF (2002) Dietary salt supplementation selectively downregulates NPR-C receptor expression in kidney independently of ANP. Am J Physiol Renal Physiol 282:F220–F227. doi:10.1152/ajprenal.0166.2001
Hartmann M, Skryabin BV, Muller T, Gazinski A, Schroter J, Gassner B, Nikolaev VO, Bunemann M, Kuhn M (2008) Alternative splicing of the guanylyl cyclase-A receptor modulates atrial natriuretic peptide signaling. J Biol Chem 283:28313–28320. doi:10.1074/jbc.M805521200
Francoeur F, Gossard F, Hamet P, Tremblay J (1995) Alternative splicing of natriuretic peptide A and B receptor transcripts in the rat brain. Clin Exp Pharmacol Physiol Suppl 22:S172–S174
Drewett JG, Fendly BM, Garbers DL, Lowe DG (1995) Natriuretic peptide receptor-B (guanylyl cyclase-B) mediates C-type natriuretic peptide relaxation of precontracted rat aorta. J Biol Chem 270:4668–4674. doi:10.1074/jbc.270.9.4668
Rapoport RM, Waldman SA, Schwartz K, Winquist RJ, Murad F (1985) Effects of atrial natriuretic factor, sodium nitroprusside, and acetylcholine on cyclic GMP levels and relaxation in rat aorta. Eur J Pharmacol 115:219–229
Lincoln TM (1983) Effects of nitroprusside and 8-bromo-cyclic GMP on the contractile activity of the rat aorta. J Pharmacol Exp Ther 224:100–107
Kamm KE, Stull JT (1985) The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol 25:593–620. doi:10.1146/annurev.pa.25.040185.003113
Somlyo AP, Somlyo AV (1994) Signal transduction and regulation in smooth muscle. Nature 372:231–236. doi:10.1038/372231a0
Somlyo AP, Himpens B (1989) Cell calcium and its regulation in smooth muscle. FASEB J 3:2266–2276
Hirano K, Phan BC, Hartshorne DJ (1997) Interactions of the subunits of smooth muscle myosin phosphatase. J Biol Chem 272:3683–3688. doi:10.1074/jbc.272.6.3683
Shimizu H, Ito M, Miyahara M, Ichikawa K, Okubo S, Konishi T, Naka M, Tanaka T, Hirano K, Hartshorne DJ (1994) Characterization of the myosin-binding subunit of smooth muscle myosin phosphatase. J Biol Chem 269:30407–30411
Hathaway DR, Adelstein RS (1979) Human platelet myosin light chain kinase requires the calcium-binding protein calmodulin for activity. Proc Natl Acad Sci USA 76:1653–1657
Pfeifer A, Klatt P, Massberg S, Ny L, Sausbier M, Hirneiss C, Wang GX, Korth M, Aszodi A, Andersson KE, Krombach F, Mayerhofer A, Ruth P, Fassler R, Hofmann F (1998) Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J 17:3045–3051. doi:10.1093/emboj/17.11.3045
Alioua A, Tanaka Y, Wallner M, Hofmann F, Ruth P, Meera P, Toro L (1998) The large conductance, voltage-dependent, and calcium-sensitive K+ channel, Hslo, is a target of cGMP-dependent protein kinase phosphorylation in vivo. J Biol Chem 273:32950–32956. doi:10.1074/jbc.273.49.32950
Swayze RD, Braun AP (2001) A catalytically inactive mutant of type I cGMP-dependent protein kinase prevents enhancement of large conductance, calcium-sensitive K+ channels by sodium nitroprusside and cGMP. J Biol Chem 276:19729–19737. doi:10.1074/jbc.M005711200
Nelson MT, Quayle JM (1995) Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol 268:C799–C822
Jiang LH, Gawler DJ, Hodson N, Milligan CJ, Pearson HA, Porter V, Wray D (2000) Regulation of cloned cardiac L-type calcium channels by cGMP-dependent protein kinase. J Biol Chem 275:6135–6143. doi:10.1074/jbc.275.9.6135
Gonzalez JM, Jost LJ, Rouse D, Suki WN (1996) Plasma membrane and sarcoplasmic reticulum Ca-ATPase and smooth muscle. Miner Electrolyte Metab 22:345–348
Cornwell TL, Pryzwansky KB, Wyatt TA, Lincoln TM (1991) Regulation of sarcoplasmic reticulum protein phosphorylation by localized cyclic GMP-dependent protein kinase in vascular smooth muscle cells. Mol Pharmacol 40:923–931
Komalavilas P, Lincoln TM (1996) Phosphorylation of the inositol 1,4,5-trisphosphate receptor. Cyclic GMP-dependent protein kinase mediates cAMP and cGMP dependent phosphorylation in the intact rat aorta. J Biol Chem 271:21933–21938. doi:10.1074/jbc.271.36.21933
Schlossmann J, Ammendola A, Ashman K, Zong X, Huber A, Neubauer G, Wang GX, Allescher HD, Korth M, Wilm M, Hofmann F, Ruth P (2000) Regulation of intracellular calcium by a signalling complex of IRAG, IP3 receptor and cGMP kinase Ibeta. Nature 404:197–201. doi:10.1038/35004606
Nakamura M, Ichikawa K, Ito M, Yamamori B, Okinaka T, Isaka N, Yoshida Y, Fujita S, Nakano T (1999) Effects of the phosphorylation of myosin phosphatase by cyclic GMP-dependent protein kinase. Cell Signal 11:671–676. doi:10.1016/S0898-6568(99)00036-4
Surks HK, Mochizuki N, Kasai Y, Georgescu SP, Tang KM, Ito M, Lincoln TM, Mendelsohn ME (1999) Regulation of myosin phosphatase by a specific interaction with cGMP-dependent protein kinase Ialpha. Science 286:1583–1587. doi:10.1126/science.286.5444.1583
Marin-Grez M, Fleming JT, Steinhausen M (1986) Atrial natriuretic peptide causes pre-glomerular vasodilatation and post-glomerular vasoconstriction in rat kidney. Nature 324:473–476. doi:10.1038/324473a0
Tremblay J, Gerzer R, Vinay P, Pang SC, Beliveau R, Hamet P (1985) The increase of cGMP by atrial natriuretic factor correlates with the distribution of particulate guanylate cyclase. FEBS Lett 181:17–22. doi:10.1016/0014-5793(85)81105-4
Sano T, Morishita Y, Matsuda Y, Yamada K (1992) Pharmacological profile of HS-142-1, a novel nonpeptide atrial natriuretic peptide antagonist of microbial origin. I. Selective inhibition of the actions of natriuretic peptides in anesthetized rats. J Pharmacol Exp Ther 260:825–831
Norling LL, Geldern TV, Chevalier RL (1994) Maturation of A71915-dependent inhibition of atrial natriuretic peptide-stimulated cyclic GMP production in isolated rat glomeruli. Biol Neonate 66:294–301
Harris PJ, Thomas D, Morgan TO (1987) Atrial natriuretic peptide inhibits angiotensin-stimulated proximal tubular sodium and water reabsorption. Nature 326:697–698. doi:10.1038/326697a0
Light DB, Corbin JD, Stanton BA (1990) Dual ion-channel regulation by cyclic GMP and cyclic GMP-dependent protein kinase. Nature 344:336–339. doi:10.1038/344336a0
Burnett JC Jr, Granger JP, Opgenorth TJ (1984) Effects of synthetic atrial natriuretic factor on renal function and renin release. Am J Physiol 247:F863–F866
Gambaryan S, Wagner C, Smolenski A, Walter U, Poller W, Haase W, Kurtz A, Lohmann SM (1998) Endogenous or overexpressed cGMP-dependent protein kinases inhibit cAMP-dependent renin release from rat isolated perfused kidney, microdissected glomeruli, and isolated juxtaglomerular cells. Proc Natl Acad Sci USA 95:9003–9008
de Lean A, Racz K, Gutkowska J, Nguyen TT, Cantin M, Genest J (1984) Specific receptor-mediated inhibition by synthetic atrial natriuretic factor of hormone-stimulated steroidogenesis in cultured bovine adrenal cells. Endocrinology 115:1636–1638
Chartier L, Schiffrin E, Thibault G, Garcia R (1984) Atrial natriuretic factor inhibits the stimulation of aldosterone secretion by angiotensin II, ACTH and potassium in vitro and angiotensin II-induced steroidogenesis in vivo. Endocrinology 115:2026–2028
Kudo T, Baird A (1984) Inhibition of aldosterone production in the adrenal glomerulosa by atrial natriuretic factor. Nature 312:756–757
MacFarland RT, Zelus BD, Beavo JA (1991) High concentrations of a cGMP-stimulated phosphodiesterase mediate ANP-induced decreases in cAMP and steroidogenesis in adrenal glomerulosa cells. J Biol Chem 266:136–142
Cherradi N, Brandenburger Y, Rossier MF, Vallotton MB, Stocco DM, Capponi AM (1998) Atrial natriuretic peptide inhibits calcium-induced steroidogenic acute regulatory protein gene transcription in adrenal glomerulosa cells. Mol Endocrinol 12:962–972
Calle RA, Bollag WB, White S, Betancourt-Calle S, Kent P (2001) ANPs effect on MARCKS and StAR phosphorylation in agonist-stimulated glomerulosa cells. Mol Cell Endocrinol 177:71–79. doi:10.1016/S0303-7207(01)00454-3
Dulak J, Jozkowicz A, mbinska-Kiec A, Guevara I, Zdzienicka A, Zmudzinska-Grochot D, Florek I, Wojtowicz A, Szuba A, Cooke JP (2000) Nitric oxide induces the synthesis of vascular endothelial growth factor by rat vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 20:659–666
Chin K, Kurashima Y, Ogura T, Tajiri H, Yoshida S, Esumi H (1997) Induction of vascular endothelial growth factor by nitric oxide in human glioblastoma and hepatocellular carcinoma cells. Oncogene 15:437–442
Hood J, Granger HJ (1998) Protein kinase G mediates vascular endothelial growth factor-induced Raf-1 activation and proliferation in human endothelial cells. J Biol Chem 273:23504–23508. doi:10.1074/jbc.273.36.23504
Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK (2001) Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci USA 98:2604–2609
Kohno M, Ikeda M, Johchi M, Horio T, Yasunari K, Kurihara N, Takeda T (1993) Interaction of PDGF and natriuretic peptides on mesangial cell proliferation and endothelin secretion. Am J Physiol 265:E673–E679
Itoh H, Pratt RE, Dzau VJ (1990) Atrial natriuretic polypeptide inhibits hypertrophy of vascular smooth muscle cells. J Clin Invest 86:1690–1697. doi:10.1172/JCI114893
Itoh H, Pratt RE, Ohno M, Dzau VJ (1992) Atrial natriuretic polypeptide as a novel antigrowth factor of endothelial cells. Hypertension 19:758–761
Cao L, Gardner DG (1995) Natriuretic peptides inhibit DNA synthesis in cardiac fibroblasts. Hypertension 25:227–234
Pedram A, Razandi M, Kehrl J, Levin ER (2000) Natriuretic peptides inhibit G protein activation. Mediation through cross-talk between cyclic GMP-dependent protein kinase and regulators of G protein-signaling proteins. J Biol Chem 275:7365–7372. doi:10.1074/jbc.275.10.7365
Suhasini M, Li H, Lohmann SM, Boss GR, Pilz RB (1998) Cyclic-GMP-dependent protein kinase inhibits the Ras/mitogen-activated protein kinase pathway. Mol Cell Biol 18:6983–6994
Furuya M, Yoshida M, Hayashi Y, Ohnuma N, Minamino N, Kangawa K, Matsuo H (1991) C-type natriuretic peptide is a growth inhibitor of rat vascular smooth muscle cells. Biochem Biophys Res Commun 177:927–931. doi:10.1016/0006-291X(91)90627-J
Furuya M, Aisaka K, Miyazaki T, Honbou N, Kawashima K, Ohno T, Tanaka S, Minamino N, Kangawa K, Matsuo H (1993) C-type natriuretic peptide inhibits intimal thickening after vascular injury. Biochem Biophys Res Commun 193:248–253. doi:10.1006/bbrc.1993.1616
Shinomiya M, Tashiro J, Saito Y, Yoshida S, Furuya M, Oka N, Tanaka S, Kangawa K, Matsuo H (1994) C-type natriuretic peptide inhibits intimal thickening of rabbit carotid artery after balloon catheter injury. Biochem Biophys Res Commun 205:1051–1056. doi:10.1006/bbrc.1994.2772
Ueno H, Haruno A, Morisaki N, Furuya M, Kangawa K, Takeshita A, Saito Y (1997) Local expression of C-type natriuretic peptide markedly suppresses neointimal formation in rat injured arteries through an autocrine/paracrine loop. Circulation 96:2272–2279
Horio T, Tokudome T, Maki T, Yoshihara F, Suga S, Nishikimi T, Kojima M, Kawano Y, Kangawa K (2003) Gene expression, secretion, and autocrine action of C-type natriuretic peptide in cultured adult rat cardiac fibroblasts. Endocrinology 144:2279–2284
Kong X, Wang X, Xu W, Behera S, Hellermann G, Kumar A, Lockey RF, Mohapatra S, Mohapatra SS (2008) Natriuretic peptide receptor a as a novel anticancer target. Cancer Res 68:249–256. doi:10.1158/0008-5472
Pollman MJ, Yamada T, Horiuchi M, Gibbons GH (1996) Vasoactive substances regulate vascular smooth muscle cell apoptosis. Countervailing influences of nitric oxide and angiotensin II. Circ Res 79:748–756
Wu CF, Bishopric NH, Pratt RE (1997) Atrial natriuretic peptide induces apoptosis in neonatal rat cardiac myocytes. J Biol Chem 272:14860–14866. doi:10.1074/jbc.272.23.14860
Taimor G, Hofstaetter B, Piper HM (2000) Apoptosis induction by nitric oxide in adult cardiomyocytes via cGMP-signaling and its impairment after simulated ischemia. Cardiovasc Res 45:588–594. doi:10.1016/S0008-6363(99)00272-2
Suenobu N, Shichiri M, Iwashina M, Marumo F, Hirata Y (1999) Natriuretic peptides and nitric oxide induce endothelial apoptosis via a cGMP-dependent mechanism. Arterioscler Thromb Vasc Biol 19:140–146
Soh JW, Mao Y, Liu L, Thompson WJ, Pamukcu R, Weinstein IB (2001) Protein kinase G activates the JNK1 pathway via phosphorylation of MEKK1. J Biol Chem 276:16406–16410. doi:10.1074/jbc.C100079200
Soh JW, Mao Y, Kim MG, Pamukcu R, Li H, Piazza GA, Thompson WJ, Weinstein IB (2000) Cyclic GMP mediates apoptosis induced by sulindac derivatives via activation of c-Jun NH2-terminal kinase 1. Clin Cancer Res 6:4136–4141
Li H, Liu L, David ML, Whitehead CM, Chen M, Fetter JR, Sperl GJ, Pamukcu R, Thompson WJ (2002) Pro-apoptotic actions of exisulind and CP461 in SW480 colon tumor cells involve beta-catenin and cyclin D1 down-regulation. Biochem Pharmacol 64:1325–1336. doi:10.1016/S0006-2952(02)01345-X
Ciani E, Guidi S, Bartesaghi R, Contestabile A (2002) Nitric oxide regulates cGMP-dependent cAMP-responsive element binding protein phosphorylation and Bcl-2 expression in cerebellar neurons: implication for a survival role of nitric oxide. J Neurochem 82:1282–1289. doi:10.1046/j.1471-4159.2002.01080.x
Zamora R, Alarcon L, Vodovotz Y, Betten B, Kim PK, Gibson KF, Billiar TR (2001) Nitric oxide suppresses the expression of Bcl-2 binding protein BNIP3 in hepatocytes. J Biol Chem 276:46887–46895. doi:10.1074/jbc.M101865200
Sengenes C, Zakaroff-Girard A, Moulin A, Berlan M, Bouloumie A, Lafontan M, Galitzky J (2002) Natriuretic peptide-dependent lipolysis in fat cells is a primate specificity. Am J Physiol Regul Integr Comp Physiol 283:R257–R265. doi:10.1152/ajpregu.00453.2001
Sengenes C, Bouloumie A, Hauner H, Berlan M, Busse R, Lafontan M, Galitzky J (2003) Involvement of a cGMP-dependent pathway in the natriuretic peptide-mediated hormone-sensitive lipase phosphorylation in human adipocytes. J Biol Chem 278:48617–48626. doi:10.1074/jbc.M303713200
Carey GB (1998) Mechanisms regulating adipocyte lipolysis. Adv Exp Med Biol 441:157–170
Conti M, Beavo J (2007) Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem 76:481–511. doi:10.1146/annurev.biochem.76.060305.150444
Sarzani R, Marcucci P, Salvi F, Bordicchia M, Espinosa E, Mucci L, Lorenzetti B, Minardi D, Muzzonigro G, Dessi-Fulgheri P, Rappelli A (2008) Angiotensin II stimulates and atrial natriuretic peptide inhibits human visceral adipocyte growth. Int J Obes (Lond) 32:259–267. doi:10.1038/sj.ijo.0803724
Jones BH, Standridge MK, Moustaid N (1997) Angiotensin II increases lipogenesis in 3T3-L1 and human adipose cells. Endocrinology 138:1512–1519
Darimont C, Vassaux G, Ailhaud G, Negrel R (1994) Differentiation of preadipose cells: paracrine role of prostacyclin upon stimulation of adipose cells by angiotensin-II. Endocrinology 135:2030–2036
Shi Y, Burn P (2004) Lipid metabolic enzymes: emerging drug targets for the treatment of obesity. Nat Rev Drug Discov 3:695–710. doi:10.1038/nrd1469
Touyz RM, Schiffrin EL (2000) Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev 52:639–672
Xue B, Greenberg AG, Kraemer FB, Zemel MB (2001) Mechanism of intracellular calcium ([Ca2+]i) inhibition of lipolysis in human adipocytes. FASEB J 15:2527–2529. doi:10.1096/fj.01-0278fje
Garg R, Pandey KN (2003) Angiotensin II-mediated negative regulation of Npr1 promoter activity and gene transcription. Hypertension 41:730–736. doi:10.1161/01.HYP.0000051890.68573.94
Belo NO, Sairam MR, Dos Reis AM (2008) Impairment of the natriuretic peptide system in follitropin receptor knockout mice and reversal by estradiol: implications for obesity-associated hypertension in menopause. Endocrinology 149:1399–1406. doi:10.1210/en.2007-0572
Moro C, Polak J, Hejnova J, Klimcakova E, Crampes F, Stich V, Lafontan M, Berlan M (2006) Atrial natriuretic peptide stimulates lipid mobilization during repeated bouts of endurance exercise. Am J Physiol Endocrinol Metab 290:E864–E869. doi:10.1152/ajpendo.00348.2005
Perez-Matute P, Neville MJ, Tan GD, Frayn KN, Karpe F (2009) Transcriptional control of human adipose tissue blood flow. Obesity (Silver Spring) 17:681–688. doi:10.1038/oby.2008.606
Birkenfeld AL, Budziarek P, Boschmann M, Moro C, Adams F, Franke G, Berlan M, Marques MA, Sweep FC, Luft FC, Lafontan M, Jordan J (2008) Atrial natriuretic peptide induces postprandial lipid oxidation in humans. Diabetes 57:3199–3204. doi:10.2337/db08-0649
Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO (2003) Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science 299:896–899. doi:10.1126/science.1079368
Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW, Vasan RS (2004) Impact of obesity on plasma natriuretic peptide levels. Circulation 109:594–600. doi:10.1161/01.CIR.0000112582.16683.EA
Rubattu S, Sciarretta S, Ciavarell GM, Venturelli V, De PP, Tocci G, De BL, Ferrucci A, Volpe M (2007) Reduced levels of N-terminal-proatrial natriuretic peptide in hypertensive patients with metabolic syndrome and their relationship with left ventricular mass. J Hypertens 25:833–839. doi:10.1097/HJH.0b013e32803cae3c
Olsen MH, Hansen TW, Christensen MK, Gustafsson F, Rasmussen S, Wachtell K, Borch-Johnsen K, Ibsen H, Jorgensen T, Hildebrandt P (2005) N-terminal pro brain natriuretic peptide is inversely related to metabolic cardiovascular risk factors and the metabolic syndrome. Hypertension 46:660–666. doi:10.1161/01.HYP.0000179575.13739.72
Dessi-Fulgheri P, Sarzani R, Tamburrini P, Moraca A, Espinosa E, Cola G, Giantomassi L, Rappelli A (1997) Plasma atrial natriuretic peptide and natriuretic peptide receptor gene expression in adipose tissue of normotensive and hypertensive obese patients. J Hypertens 15:1695–1699
Sarzani R, Paci VM, Zingaretti CM, Pierleoni C, Cinti S, Cola G, Rappelli A, Dessi-Fulgheri P (1995) Fasting inhibits natriuretic peptides clearance receptor expression in rat adipose tissue. J Hypertens 13:1241–1246
Dessi-Fulgheri P, Sarzani R, Serenelli M, Tamburrini P, Spagnolo D, Giantomassi L, Espinosa E, Rappelli A (1999) Low calorie diet enhances renal, hemodynamic, and humoral effects of exogenous atrial natriuretic peptide in obese hypertensives. Hypertension 33:658–662
Wang TJ, Larson MG, Keyes MJ, Levy D, Benjamin EJ, Vasan RS (2007) Association of plasma natriuretic peptide levels with metabolic risk factors in ambulatory individuals. Circulation 115:1345–1353. doi:10.1161/CIRCULATIONAHA.106.655142
Verspohl EJ, Bernemann IK (1996) Atrial natriuretic peptide (ANP)-induced inhibition of glucagon secretion: mechanism of action in isolated rat pancreatic islets. Peptides 17:1023–1029. doi:10.1016/0196-9781(96)00152-0
Steinhelper ME, Cochrane KL, Field LJ (1990) Hypotension in transgenic mice expressing atrial natriuretic factor fusion genes. Hypertension 16:301–307
John SW, Krege JH, Oliver PM, Hagaman JR, Hodgin JB, Pang SC, Flynn TG, Smithies O (1995) Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science 267:679–681. doi:10.1126/science.7839143
Wang D, Oparil S, Feng JA, Li P, Perry G, Chen LB, Dai M, John SW, Chen YF (2003) Effects of pressure overload on extracellular matrix expression in the heart of the atrial natriuretic peptide-null mouse. Hypertension 42:88–95. doi:10.1161/01.CIR.0000112582.16683.EA
Honrath U, Chong CK, Melo LG, Sonnenberg H (1999) Effect of saline infusion on kidney and collecting duct function in atrial natriuretic peptide (ANP) gene “knockout” mice. Can J Physiol Pharmacol 77:454–457
Oliver PM, John SW, Purdy KE, Kim R, Maeda N, Goy MF, Smithies O (1998) Natriuretic peptide receptor 1 expression influences blood pressures of mice in a dose-dependent manner. Proc Natl Acad Sci USA 95:2547–2551
Oliver PM, Fox JE, Kim R, Rockman HA, Kim HS, Reddick RL, Pandey KN, Milgram SL, Smithies O, Maeda N (1997) Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc Natl Acad Sci USA 94:14730–14735
Lopez MJ, Wong SK, Kishimoto I, Dubois S, Mach V, Friesen J, Garbers DL, Beuve A (1995) Salt-resistant hypertension in mice lacking the guanylyl cyclase-A receptor for atrial natriuretic peptide. Nature 378:65–68. doi:10.1038/378065a0
Kilic A, Velic A, De Windt LJ, Fabritz L, Voss M, Mitko D, Zwiener M, Baba HA, Eickels MV, Schlatter E, Kuhn M (2005) Enhanced activity of the myocardial Na+/H+ exchanger NHE-1 contributes to cardiac remodeling in atrial natriuretic peptide receptor-deficient mice. Circulation 112:2307–2317. doi:10.1161/CIRCULATIONAHA.105.542209
Holtwick R, Eickels MV, Skryabin BV, Baba HA, Bubikat A, Begrow F, Schneider MD, Garbers DL, Kuhn M (2003) Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-A. J Clin Invest 111:1399–1407. doi:10.1172/JCI17061
Holtwick R, Gotthardt M, Skryabin B, Steinmetz M, Potthast R, Zetsche B, Hammer RE, Herz J, Kuhn M (2002) Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc Natl Acad Sci USA 99:7142–7147. doi:10.1073/pnas.102650499
Sabrane K, Kruse MN, Fabritz L, Zetsche B, Mitko D, Skryabin BV, Zwiener M, Baba HA, Yanagisawa M, Kuhn M (2005) Vascular endothelium is critically involved in the hypotensive and hypovolemic actions of atrial natriuretic peptide. J Clin Invest 115:1666–1674. doi:10.1172/JCI23360
Kishimoto I, Rossi K, Garbers DL (2001) A genetic model provides evidence that the receptor for atrial natriuretic peptide (guanylyl cyclase-A) inhibits cardiac ventricular myocyte hypertrophy. Proc Natl Acad Sci USA 98:2703–2706. doi:10.1073/pnas.051625598
Ogawa Y, Itoh H, Tamura N, Suga S, Yoshimasa T, Uehira M, Matsuda S, Shiono S, Nishimoto H, Nakao K (1994) Molecular cloning of the complementary DNA and gene that encode mouse brain natriuretic peptide and generation of transgenic mice that overexpress the brain natriuretic peptide gene. J Clin Invest 93:1911–1921. doi:10.1172/JCI117182
Suda M, Ogawa Y, Tanaka K, Tamura N, Yasoda A, Takigawa T, Uehira M, Nishimoto H, Itoh H, Saito Y, Shiota K, Nakao K (1998) Skeletal overgrowth in transgenic mice that overexpress brain natriuretic peptide. Proc Natl Acad Sci USA 95:2337–2342
Tamura N, Ogawa Y, Chusho H, Nakamura K, Nakao K, Suda M, Kasahara M, Hashimoto R, Katsuura G, Mukoyama M, Itoh H, Saito Y, Tanaka I, Otani H, Katsuki M (2000) Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc Natl Acad Sci USA 97:4239–4244
Chusho H, Tamura N, Ogawa Y, Yasoda A, Suda M, Miyazawa T, Nakamura K, Nakao K, Kurihara T, Komatsu Y, Itoh H, Tanaka K, Saito Y, Katsuki M, Nakao K (2001) Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc Natl Acad Sci USA 98:4016–4021. doi:10.1073/pnas.071389098
Yasoda A, Komatsu Y, Chusho H, Miyazawa T, Ozasa A, Miura M, Kurihara T, Rogi T, Tanaka S, Suda M, Tamura N, Ogawa Y, Nakao K (2004) Overexpression of CNP in chondrocytes rescues achondroplasia through a MAPK-dependent pathway. Nat Med 10:80–86. doi:10.1038/nm971
Tamura N, Doolittle LK, Hammer RE, Shelton JM, Richardson JA, Garbers DL (2004) Critical roles of the guanylyl cyclase B receptor in endochondral ossification and development of female reproductive organs. Proc Natl Acad Sci USA 101:17300–17305. doi:10.1073/pnas.0407894101
Langenickel TH, Buttgereit J, Pagel-Langenickel I, Lindner M, Monti J, Beuerlein K, Al-Saadi N, Plehm R, Popova E, Tank J, Dietz R, Willenbrock R, Bader M (2006) Cardiac hypertrophy in transgenic rats expressing a dominant-negative mutant of the natriuretic peptide receptor B. Proc Natl Acad Sci USA 103:4735–4740. doi:10.1073/pnas.0510019103
Matsukawa N, Grzesik WJ, Takahashi N, Pandey KN, Pang S, Yamauchi M, Smithies O (1999) The natriuretic peptide clearance receptor locally modulates the physiological effects of the natriuretic peptide system. Proc Natl Acad Sci USA 96:7403–7408
Dutil J, Deng AY (2001) Further chromosomal mapping of a blood pressure QTL in Dahl rats on chromosome 2 using congenic strains. Physiol Genomics 6:3–9
Pausova Z, Gaudet D, Gossard F, Bernard M, Kaldunski ML, Jomphe M, Tremblay J, Hudson TJ, Bouchard G, Kotchen TA, Cowley AW, Hamet P (2005) Genome-wide scan for linkage to obesity-associated hypertension in French Canadians. Hypertension 46:1280–1285. doi:10.1161/01.HYP.0000188049.23233.fb
Hamet P, Merlo E, Seda O, Broeckel U, Tremblay J, Kaldunski M, Gaudet D, Bouchard G, Deslauriers B, Gagnon F, Antoniol G, Pausova Z, Labuda M, Jomphe M, Gossard F, Tremblay G, Kirova R, Tonellato P, Orlov SN, Pintos J, Platko J, Hudson TJ, Rioux JD, Kotchen TA, Cowley AW Jr (2005) Quantitative founder-effect analysis of French Canadian families identifies specific loci contributing to metabolic phenotypes of hypertension. Am J Hum Genet 76:815–832. doi:10.1086/430133
Newton-Cheh C, Larson MG, Vasan RS, Levy D, Bloch KD, Surti A, Guiducci C, Kathiresan S, Benjamin EJ, Struck J, Morgenthaler NG, Bergmann A, Blankenberg S, Kee F, Nilsson P, Yin X, Peltonen L, Vartiainen E, Salomaa V, Hirschhorn JN, Melander O, Wang TJ (2009) Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet 41:348–353. doi:10.1038/ng.328
Burnett JC Jr, Kao PC, Hu DC, Heser DW, Heublein D, Granger JP, Opgenorth TJ, Reeder GS (1986) Atrial natriuretic peptide elevation in congestive heart failure in the human. Science 231:1145–1147. doi:10.1126/science.2935937
Yandle TG, Richards AM, Gilbert A, Fisher S, Holmes S, Espiner EA (1993) Assay of brain natriuretic peptide (BNP) in human plasma: evidence for high molecular weight BNP as a major plasma component in heart failure. J Clin Endocrinol Metab 76:832–838
Richards AM, Lainchbury JG, Troughton RW, Espiner EA, Nicholls MG (2004) Clinical applications of B-type natriuretic peptides. Trends Endocrinol Metab 15:170–174. doi:10.1016/j.tem.2004.03.005
Makikallio AM, Makikallio TH, Korpelainen JT, Vuolteenaho O, Tapanainen JM, Ylitalo K, Sotaniemi KA, Huikuri HV, Myllyla VV (2005) Natriuretic peptides and mortality after stroke. Stroke 36:1016–1020. doi:10.1161/01.STR.0000162751.54349.ae
Wazni OM, Martin DO, Marrouche NF, Latif AA, Ziada K, Shaaraoui M, Almahameed S, Schweikert RA, Saliba WI, Gillinov AM, Tang WH, Mills RM, Francis GS, Young JB, Natale A (2004) Plasma B-type natriuretic peptide levels predict postoperative atrial fibrillation in patients undergoing cardiac surgery. Circulation 110:124–127. doi:10.1161/01.CIR.0000134481.24511.BC
Doust JA, Pietrzak E, Dobson A, Glasziou P (2005) How well does B-type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. BMJ 330:625–634. doi:10.1136/bmj.330.7492.625
Cusson JR, Thibault G, Cantin M, Larochelle P (1990) Prolonged low dose infusion of atrial natriuretic factor in essential hypertension. Clin Exp Hypertens A 12:111–135
Hirata Y, Ishii M, Sugimoto T, Matsuoka H, Fukui K, Sugimoto T, Yamakado M, Tagawa H, Miyata A, Kangawa K et al (1988) Hormonal and renal effects of atrial natriuretic peptide in patients with secondary hypertension. Circulation 78:1401–1410
Hamet P, Testaert E, Palmour R, Larochelle P, Cantin M, Gutkowska J, Langlois Y, Ervin F, Tremblay J (1989) Effect of prolonged infusion of ANF in normotensive and hypertensive monkeys. Am J Hypertens 2:690–695
Lin KF, Chao J, Chao L (1995) Human atrial natriuretic peptide gene delivery reduces blood pressure in hypertensive rats. Hypertension 26:847–853
Cody RJ, Atlas SA, Laragh JH, Kubo SH, Covit AB, Ryman KS, Shaknovich A, Pondolfino K, Clark M, Camargo MJ et al (1986) Atrial natriuretic factor in normal subjects and heart failure patients. Plasma levels and renal, hormonal, and hemodynamic responses to peptide infusion. J Clin Invest 78:1362–1374. doi:10.1172/JCI112723
Fifer MA, Molina CR, Quiroz AC, Giles TD, Herrmann HC, Scheeder ID, Clement DL, Kubo S, Cody RJ, Cohn JN et al (1990) Hemodynamic and renal effects of atrial natriuretic peptide in congestive heart failure. Am J Cardiol 65:211–216
Weder AB, Sekkarie MA, Takiyyuddin M, Schork NJ, Julius S (1987) Antihypertensive and hypotensive effects of atrial natriuretic factor in men. Hypertension 10:582–589
Kitashiro S, Sugiura T, Takayama Y, Tsuka Y, Izuoka T, Tokunaga S, Iwasaka T (1999) Long-term administration of atrial natriuretic peptide in patients with acute heart failure. J Cardiovasc Pharmacol 33:948–952
Hobbs RE, Miller LW, Bott-Silverman C, James KB, Rincon G, Grossbard EB (1996) Hemodynamic effects of a single intravenous injection of synthetic human brain natriuretic peptide in patients with heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 78:896–901. doi:10.1016/S0002-9149(96)00464-X
Colucci WS, Elkayam U, Horton DP, Abraham WT, Bourge RC, Johnson AD, Wagoner LE, Givertz MM, Liang CS, Neibaur M, Haught WH, LeJemtel TH (2000) Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide Study Group. N Engl J Med 343:246–253
Rayburn BK, Bourge RC (2001) Nesiritide: a unique therapeutic cardiac peptide. Rev Cardiovasc Med 2(Suppl 2):S25–S31
Sackner-Bernstein JD, Kowalski M, Fox M, Aaronson K (2005) Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. JAMA 293:1900–1905
Sackner-Bernstein JD, Skopicki HA, Aaronson KD (2005) Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation 111:1487–1491. doi:10.1161/01.CIR.0000159340.93220.E4
Corti R, Burnett JC Jr, Rouleau JL, Ruschitzka F, Luscher TF (2001) Vasopeptidase inhibitors: a new therapeutic concept in cardiovascular disease? Circulation 104:1856–1862. doi:10.1161/hc4001.097191
Campese VM, Lasseter KC, Ferrario CM, Smith WB, Ruddy MC, Grim CE, Smith RD, Vargas R, Habashy MF, Vesterqvist O, Delaney CL, Liao WC (2001) Omapatrilat versus lisinopril: efficacy and neurohormonal profile in salt-sensitive hypertensive patients. Hypertension 38:1342–1348. doi:10.1161/hy1201.096569
Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E (2004) Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens 17:103–111
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martel, G., Hamet, P. & Tremblay, J. Central role of guanylyl cyclase in natriuretic peptide signaling in hypertension and metabolic syndrome. Mol Cell Biochem 334, 53–65 (2010). https://doi.org/10.1007/s11010-009-0326-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0326-8