Abstract
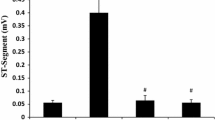
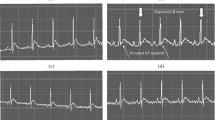
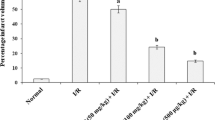
In this study, effects of Lacidipine (LAC), Ramipril (RAM) and Valsartan (VAL) on DNA damage and oxidative stress occurred in acute and chronic periods after isoproterenol (ISO)-induced myocardial infarct (MI) were investigated in rats. LAC, RAM and VAL had been administered by oral gavage at 3, 3 and 30 mg/kg doses, respectively, in acute and chronic periods following MI. In acute MI model, LAC, RAM and VAL had been administered once per day to rat groups during 30 days. On days 29 and 30, the rats of the acute MI control and drug treatment groups were administered 180 mg/kg ISO, subcutaneously at an interval of 24 h. In chronic MI model, LAC, RAM and VAL had been administered to rat groups during 30 days, and on the 1st and 2nd days, the rats of the chronic MI control and drug treatment groups were administered ISO, by the same way. After this period, routine biochemistry indicators of MI, alanin aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), creatine kinase (CK), creatine kinase-isoenzymes (CK-MB), troponin I (TnI) and nitric oxide (NO), oxidative stress indicator, has been measured in the serums obtained from rat’s blood. Also, 7,8-Dihydro-8-oxo-guanine (8-OHGua), which is an indicator of DNA damage level, has been determined in whole blood. After MI diagnosis, the relationships among the 8-OHGua, NO and clinic MI indicators have been determined. Results have been evaluated by comparing with that of control group. In control groups, the clinic MI indicators have been found to be statistically higher than the drug groups. In parallel to this increase in MI indicators, there have been determined a significant decrease in NO levels and an increase in 8-OHGua level. There was no significant difference in the rat groups which received drugs without MI induction. We have observed that the level of 8-OHGua which increased after MI in both acute and chronic periods decreased by LAC, RAM and VAL when compared to acute and chronic MI control groups. In conclusion, it has been determined that oxidative stress has been increased after ISO induced MI model and this stress reduces NO and even damages DNA. LAC, RAM and VAL may decrease the severity of MI and prevent DNA damage by reducing oxidative stress.
Similar content being viewed by others
References
Maxwell SR, Lip GY (1997) Reperfusion injury: a review of the pathophysiology, clinical manifestations and therapeutic options. Int J Cardiol 58:95–117. doi:10.1016/S0167-5273(96)02854-9
Wexler BC, Greenberg BP (1978) Protective effects of clofibrate on ısoproterenol-ınduced myocardial-ınfarction in arteriosclerotic and non-arteriosclerotic rats. Atherosclerosis 29:373–395. doi:10.1016/0021-9150(78)90084-9
Yeager JC, Iams SG (1981) The hemodynamics of isoproterenol-induced cardiac failure in the rat. Circ Shock 8:151–163
Bloom S, Davis Dl (1972) Calcium as mediator of isoproterenol-induced myocardial necrosis. Am J Pathol 69:459–470
Fleckenstein A, Janke J, Doring HJ et al (1974) Myocardial fiber necrosis due to intracellular Ca overload-a new principle in cardiac pathophysiology. Recent Adv Stud Cardiac Struct Metab 4:563–580
Sharma M, Kishore K, Gupta SK et al (2001) Cardioprotective potential of ocimum sanctum in isoproterenol induced myocardial infarction in rats. Mol Cell Biochem 225:75–83. doi:10.1023/A:1012220908636
Miwa S, Toyokuni S, Nishina T et al (2002) Spaciotemporal alteration of 8-hydroxy-2′-deoxyguanosine levels in cardiomyocytes after myocardial infarction in rats. Free Radic Res 36:853–858. doi:10.1081/1071576021000005285
Dizdaroglu M, Jaruga P, Birincioglu M et al (2002) Free radical-induced damage to DNA: mechanisms and measurement. Free Radic Biol Med 32:1102–1115. doi:10.1016/S0891-5849(02)00826-2
Kasai H, Nishimura S (1984) Hydroxylation of deoxyguanosine at the C-8 position by ascorbic acid and other reducing agents. Nucleic Acids Res 12:2137–2145. doi:10.1093/nar/12.4.2137
Kasai H (1997) Analysis of a form of oxidative DNA damage, 8-hydroxy-2′-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat Res 387:147–163. doi:10.1016/S1383-5742(97)00035-5
Floyd RA, Watson JJ, Wong PK et al (1986) Hydroxyl free radical adduct of deoxyguanosine: sensitive detection and mechanisms of formation. Free Radic Res Commun 1:163–172. doi:10.3109/10715768609083148
Van’t Hof RJ, Ralston SH (2001) Nitric oxide and bone. Immunology 103:255–261. doi:10.1046/j.1365-2567.2001.01261.x
Yucel D, Senes M, Topkaya B et al (2006) Oxidative/nitrosative stress in chronic heart failure: a critical review. Turk J Biochem 31:86–95
Hertog MG, Feskens EJ, Hollman PC et al (1993) Dietary antioxidant flavonoids and risk of coronary heart-disease—the zutphen elderly study. Lancet 342:1007–1011. doi:10.1016/0140-6736(93)92876-U
Gupta SK, Mohanty I, Talwar KK et al (2004) Cardioprotection from ischemia and reperfusion injury by Withania somnifera: a hemodynamic, biochemical and histopathological assessment. Mol Cell Biochem (260):39–47
Sumitra M, Manikandan P, Kumar DA et al (2001) Experimental myocardial necrosis in rats: role of arjunolic acid on platelet aggregation, coagulation and antioxidant status. Mol Cell Biochem 224:135–142
Kyselovic J, Krenek P, Wibo M et al (2001) Effects of amlodipine and lacidipine on cardiac remodelling and renin production in salt-loaded stroke-prone hypertensive rats. Br J Pharmacol 134:1516–1522. doi:10.1038/sj.bjp.0704398
Wolkart G, Pang X, Stessel H et al (2007) Chronic endothelin-A receptor antagonism is as protective as angiotensin converting enzyme inhibition against cardiac dysfunction in diabetic rats. Br J Pharmacol 151:1187–1197. doi:10.1038/sj.bjp.0707325
Cm Y, Wing-Hon LK, Li PS et al (2004) Normalization of renal aquaporin-2 water channel expression by fosinopril, valsartan, and combination therapy in congestive heart failure: a new mechanism of action. J Mol Cell Cardiol 36:445–453. doi:10.1016/j.yjmcc.2004.01.002
Rona G, Chappel CI, Balazs T et al (1959) An infarct-like myocardial lesion and other toxic manifestations produced by isoproterenol in the rat. AMA Arch Pathol 67:443–455
ECCLS (1989) Determination of catalytic concentration in serum of l-alanine aminotransferase (E.C. 2.6.1.2, ALAT). Klin Chem Mitt 20:204–211
ECCLS (1989) Determination of catalytic concentration in serum of L-aspartate aminotransferase (E.C. 2.6.1.1, ASAT). Klin Chem Mitt 20:198–204
Schumann G, Bonora R, Ceriotti F et al (2002) IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37°C. Part 3. Reference procedure for the measurement of catalytic concentration of lactate dehydrogenase. Clin Chem Lab Med 40(6):643–648
Lott JA, Stang JM (1980) Serum enzymes and isoenzymes in the diagnosis and differantial diagnosis of mycardial ischemia and necrosis. Clin Chem 26:1241–1250
Wu AH, Feng YJ, Moore R et al (1998) Characterization of cardiac troponin subunit release into serum after acute myocardial infarction and comparison of assays for troponin T and I. American Association for Clinical Chemistry Subcommittee on cTnI Standardization. Clin Chem 44(6 Pt 1):1198–1208
Moshage H, Kok B, Huizenga JR et al (1995) Nitrite and nitrate determinations in plasma: a critical evaluation. Clin Chem 41:892–896
Adeli K, Ogbonna G (1990) Rapid purification of human DNA from whole blood for potential application in clinical chemistry laboratories. Clin Chem 36:261–264
Kaur H, Halliwell B (1996) Measurement of oxidized and methylated DNA bases by HPLC with electrochemical detection. Biochem J 318(Pt 1):21–23
Sathish V, Ebenezar KK, Devaki T (2003) Synergistic effect of Nicorandil and Amlodipine on tissue defense system during experimental myocardial infarction in rats. Mol Cell Biochem 243:133–138. doi:10.1023/A:1021612230000
Jayalakshmi R, Thirupurasundari CJ, Devaraj SN (2006) Pretreatment with alcoholic extract of Crataegus oxycantha (AEC) activates mitochondrial protection during isoproterenol-induced myocardial infarction in rats. Mol Cell Biochem 292:59–67. doi:10.1007/s11010-006-9218-3
Whealthy AM, Thandroyen FT, Opie LH (1985) Catecholamine induced myocardial cell damage: catecholamines or adrenochrome. J Mol Cell Cardiol 17:349–359. doi:10.1016/S0022-2828(85)80134-6
Rajadurai M, Prince PS (2007) Preventive effect of naringin on isoproterenol-induced cardiotoxicity in Wistar rats: an in vivo and in vitro study. Toxicology 232:216–225. doi:10.1016/j.tox.2007.01.006
Brodde OE (1991) Beta 1- and beta 2-adrenoceptors in the human heart: properties, function, and alterations in chronic heart failure. Pharmacol Rev 43:203–242
Remiao F, Carmo H, Carvalho F et al (2001) Copper enhances isoproterenol toxicity in isolated rat cardiomyocytes: effects on oxidative stress. Cardiovasc Toxicol 1:195–204. doi:10.1385/CT:1:3:195
Rajadurai M, P Stanely Mainzen Prince (2006) Preventive effect of naringin on lipid peroxides and antioxidants in isoproterenol-induced cardiotoxicity in Wistar rats: biochemical and histopathological evidences. Toxicology 228:259–268. doi:10.1016/j.tox.2006.09.005
Wittevean Sagi AGJ, Hemker HC, Hollar L, Hermens W (1975) The quantification of infarct size in man by means of plasma enzyme levels. Br Heart J 37:795–803. doi:10.1136/hrt.37.8.795
Suchalatha S, Shyamaladevi CS (2004) Effect of arogh—a polyherbal formulatıon on the marker enzymes ın ısoproterenol induced myocardial ınjury. Indian J Clin Biochem 19:184–189. doi:10.1007/BF02894283
Adams JE 3rd, Bodor GS, Dávila-Román VG et al (1993) Cardiac troponin I. A marker with high specificity for cardiac injury. Circulation 88:101–106
Puleo PR, Meyer D, Wathen C et al (1994) Use of a rapid assay of subforms of creatine kinase-MB to diagnose or rule out acute myocardial infarction. N Engl J Med 331:561–566. doi:10.1056/NEJM199409013310901
Acikel M, Buyukokuroglu ME, Erdogan F et al (2005) Protective effects of dantrolene against myocardial injury induced by isoproterenol in rats: biochemical and histological findings. Int J Cardiol 98:389–394. doi:10.1016/j.ijcard.2003.10.054
Van Amsterdam FT, Roveri A, Maiorino M et al (1992) Lacidipine: a dihydropyridine calcium antagonist with antioxidant activity. Free Radic Biol Med 12:183–187. doi:10.1016/0891-5849(92)90025-C
Aslam S (2008) Cardiovascular disease in dialysis patients: do some antihypertensive drugs have specific antioxidant effects or is it just blood pressure reduction? Does antioxidant treatment reduce the risk for cardiovascular disease? Curr Opin Nephrol Hypertens 17:99–105
Lei SZ, Pan ZH, Aggarwal SK et al (1992) Effect of nitric oxide production on the redox modulatory site of the NMDA receptor-channel complex. Neuron 8:1087–1099. doi:10.1016/0896-6273(92)90130-6
Siegfried MR, Erhardt J, Rider T et al (1992) Cardioprotection and attenuation of endothelial dysfunction by organic nitric oxide donors in myocardial ischemia-reperfusion. J Pharmacol Exp Ther 260:668–675
Collins AR, Dusinska M, Gedik CM et al (1996) Oxidative damage to DNA: do we have a reliable biomarker? Environ Health Perspect 104(Suppl 3):465–469. doi:10.2307/3432805
Jornot L, Petersen H, Junod AF (1998) Hydrogen peroxide-induced DNA damage is independent of nuclear calcium but dependent on redox-active ions. Biochem J 335(Pt 1):85–94
Cooke MS, Evans MD, Dizdaroglu M et al (2003) Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J 17:1195–1214. doi:10.1096/fj.02-0752rev
Cadet J, Douki T, Gasparutto D et al (2003) Oxidative damage to DNA: formation, measurement and biochemical features. Mutat Res 531:5–23. doi:10.1016/j.mrfmmm.2003.09.001
Dizdaroglu M (1994) Chemical determination of oxidative DNA damage by gas chromatography-mass spectrometry. Methods Enzymol 234:3–16. doi:10.1016/0076-6879(94)34072-2
Shigenaga MK, Aboujaoude EN, Chen Q et al (1994) Assays of oxidative DNA damage biomarkers 8-oxo-2′-deoxyguanosine and 8-oxoguanine in nuclear DNA and biological fluids by high-performance liquid chromatography with electrochemical detection. Methods Enzymol 234:16–33. doi:10.1016/0076-6879(94)34073-0
Evans MD, Cooke MS, Podmore ID et al (1999) Discrepancies in the measurement of UVC-induced 8-oxo-2′-deoxyguanosine: implications for the analysis of oxidative DNA damage. Biochem Biophys Res Commun 259:374–378. doi:10.1006/bbrc.1999.0801
Kasai H, Nishimura S (1991) Formation of 8-hydroxydeoxyguanosine in DNA. In: Sies H (ed) Oxidative stress:oxidants and antioxidants. Academic Press, London, pp 99–116
Shigenaga MK, Gimeno CJ, Amens BN (1989) Urinary 8-hydroxy-2′-deoxyguanosine as a biological marker of in vivo oxidative DNA damage. Proc Natl Acad Sci USA 86:9697–9701. doi:10.1073/pnas.86.24.9697
Acknowledgments
The second author (Y. Bayir) wishes to thank Associated Professors Fehmi Odabasoglu and the Bachelor of sciences Elif Cadirci, Hakan Alp, Fadime Atalay, Selma Mutlu and Mesut Halici for kind support in the doctorate study.
Author information
Authors and Affiliations
Corresponding author
Additional information
This research is included in a doctorate study belongs to Yasin BAYIR, student of Ataturk University Institute of Health Sciences and was conducted in the Laboratory of Pharmacology at Ataturk University, Faculty of Medicine, Department of Pharmacology, 25240 Erzurum, Turkey, the Laboratory of Biochemistry at Ataturk University, Faculty of Pharmacy, Department of Biochemistry, 25240 Erzurum, Turkey and the Laboratory of Biochemistry at Ataturk University, Faculty of Medicine, Department of Biochemistry, 25240 Erzurum, Turkey.
Rights and permissions
About this article
Cite this article
Keles, M.S., Bayir, Y., Suleyman, H. et al. Investigation of effects of Lacidipine, Ramipril and Valsartan on DNA damage and oxidative stress occurred in acute and chronic periods following isoproterenol-induced myocardial infarct in rats. Mol Cell Biochem 328, 109–117 (2009). https://doi.org/10.1007/s11010-009-0080-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0080-y