Abstract
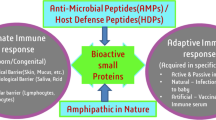
Antimicrobial peptides (AMPs) are small sized protein molecules which play a crucial role in host inborn immune framework. AMPs with their specific properties are often considered as a barrier against various harmful foreign particles including bacteria, yeast, fungi and virus. These peptides from different sources exhibit diverse functions and structural features. It exploits the unique property of membrane interaction to destroy target organisms and even cellular components are targeted. However, to combat the emerging resistance towards antibiotics, the use of AMPs as natural drugs has gained importance. Efficiency of these peptides can be enhanced by modification in different aspects through in silico approach. In this article we focus on the areas on antimicrobial peptides like source, properties, classification, mechanism of action, resistance and strategies for its applications.


Similar content being viewed by others
References
Agerberth B, Gunne H et al (1995) FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc Natl Acad Sci 92(1):195–199
Aley SB, Zimmerman M et al (1994) Killing of Giardia lamblia by cryptdins and cationic neutrophil peptides. Infect Immun 62(12):5397–5403
Amaral AC, Silva ON et al (2012) Predicting antimicrobial peptides from eukaryotic genomes: in silico strategies to develop antibiotics. Peptides 37(2):301–308
Andersson DI, Hughes D et al (2016) Mechanisms and consequences of bacterial resistance to antimicrobial peptides. Drug Resist Updates 26:43–57
Andreu D, Rivas L (1998) Animal antimicrobial peptides: an overview. Pept Sci 47(6):415–433
Arrighi RB, Nakamura C et al (2002) Design and activity of antimicrobial peptides against sporogonic-stage parasites causing murine malarias. Antimicrob Agents Chemother 46(7):2104–2110
Avrahami D, Shai Y (2002) Conjugation of a magainin analogue with lipophilic acids controls hydrophobicity, solution assembly, and cell selectivity. Biochemistry 41(7):2254–2263
Baghian A, Jaynes J et al (1997) An amphipathic α-helical synthetic peptide analogue of melittin inhibits herpes simplex virus-1 (HSV-1)-induced cell fusion and virus spread. Peptides 18(2):177–183
Bahar A, Ren D (2013) Antimicrobial peptides. Pharmaceuticals 6(12):1543–1575
Baker MA, Maloy WL et al (1993) Anticancer efficacy of Magainin2 and analogue peptides. Cancer Res 53(13):3052–3057
Ballweber L, Jaynes J et al (2002) In vitro microbicidal activities of cecropin peptides D2A21 and D4E1 and gel formulations containing 0.1 to 2% D2A21 against Chlamydia trachomatis. Antimicrob Agents Chemother 46(1):34–41
Bals R (2000) Epithelial antimicrobial peptides in host defense against infection. Respir Res 1(3):5
Bals R, Wang X et al (1999) Mouse β-defensin 3 is an inducible antimicrobial peptide expressed in the epithelia of multiple organs. Infect Immun 67(7):3542–3547
Bayramov DF, Neff JA (2017) Beyond conventional antibiotics—New directions for combination products to combat biofilm. Adv Drug Deliv Rev 112:48–60
Benincasa M, Skerlavaj B et al (2003) In vitro and in vivo antimicrobial activity of two α-helical cathelicidin peptides and of their synthetic analogs. Peptides 24(11):1723–1731
Biswaro LS, da Costa Sousa MG et al (2018) Antimicrobial peptides and nanotechnology, recent advances and challenges. Front Microbiol 9:855
Blondelle E, Lohner K (2010) Optimization and high-throughput screening of antimicrobial peptides. Curr Pharm Design 16(28):3204–3211
Boman HG (1995) Peptide antibiotics and their role in innate immunity. Annu Rev Immunol 13(1):61–92
Boman HG (2000) Innate immunity and the normal microflora. Immunol Rev 173(1):5–16
Boman HG, Nilsson I et al (1972) Inducible antibacterial defence system in Drosophila. Nature 237(5352):232
Bowdish DM, Davidson DJ et al (2004) The human cationic peptide LL-37 induces activation of the extracellular signal-regulated kinase and p38 kinase pathways in primary human monocytes. J Immunol 172(6):3758–3765
Bowdish DM, Davidson DJ et al (2005a) A re-evaluation of the role of host defence peptides in mammalian immunity. Curr Protein Pept Sci 6(1):35–51
Bowdish DM, Davidson DJ et al (2005b) Impact of LL-37 on anti‐infective immunity. J Leukoc Biol 77(4):451–459
Brogden KA (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3(3):238
Brogden KA, Ackermann M et al (2003) Antimicrobial peptides in animals and their role in host defences. Int J Antimicrob Agents 22(5):465–478
Brown KL, Hancock RE (2006) Cationic host defense (antimicrobial) peptides. Curr Opin Immunol 18(1):24–30
Bruix M, Jimenez M et al (1993) Solution structure of gamma. 1-H and. gamma. 1-P thionins from barley and wheat endosperm determined by proton NMR: a structural motif common to toxic arthropod proteins. Biochemistry 32(2):715–724
Brumfitt W, Salton MR et al (2002) Nisin, alone and combined with peptidoglycan-modulating antibiotics: activity against methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. J Antimicrob Chemother 50(5):731–734
Bryan LE (1988) General mechanisms of resistance to antibiotics. J Antimicrob Chemother 22(Supplement_A):1–15
Bulet P, Hetru C et al (1999) Antimicrobial peptides in insects; structure and function. Dev Compr Immunol 23(4–5):329–344
Chan YR, Gallo RL (1998) PR-39, a syndecan-inducing antimicrobial peptide, binds and affects p130Cas. J Biol Chem 273(44):28978–28985
Charlet M, Chernysh S et al (1996) Innate immunity isolation of several cysteine-rich antimicrobial peptides from the blood of a mollusc, Mytilus edulis. J Biol Chem 271(36):21808–21813
Chen L, Harrison S (2007) Cell-penetrating peptides in drug development: enabling intracellular targets. Portland Press, London
Chen Y, Mant CT et al (2005) Rational design of α-helical antimicrobial peptides with enhanced activities and specificity/therapeutic index. J Biol Chem 280(13):12316–12329
Conlon JM, Sonnevend A (2010) Antimicrobial peptides in frog skin secretions. Antimicrobial peptides. Springer, New York, pp 3–14
Cudic M, Otvos L Jr (2002) Intracellular targets of antibacterial peptides. Curr Drug Targets 3(2):101–106
da Cunha NB, Cobacho NB et al (2017) The next generation of antimicrobial peptides (AMPs) as molecular therapeutic tools for the treatment of diseases with social and economic impacts. Drug discovery today 22(2):234–248
da Silva FP, Machado MCC (2012) Antimicrobial peptides: clinical relevance and therapeutic implications. Peptides 36(2):308–314
Daher KA, Selsted ME et al (1986) Direct inactivation of viruses by human granulocyte defensins. J Virol 60(3):1068–1074
Dathe M, Schümann M et al (1996) Peptide helicity and membrane surface charge modulate the balance of electrostatic and hydrophobic interactions with lipid bilayers and biological membranes. Biochemistry 35(38):12612–12622
Dathe M, Wieprecht T et al (1997) Hydrophobicity, hydrophobic moment and angle subtended by charged residues modulate antibacterial and haemolytic activity of amphipathic helical peptides. FEBS Lett 403(2):208–212
de Azevedo J, Walter F et al (2008) Computational methods for calculation of ligand-binding affinity. Curr Drug Targets 9(12):1031–1039
De Caleya RF, Gonzalez-Pascual B et al (1972) Susceptibility of phytopathogenic bacteria to wheat purothionins in vitro. Appl Environ Microbiol 23(5):998–1000
De Lucca AJ, Walsh TJ (1999) Antifungal peptides: novel therapeutic compounds against emerging pathogens. Antimicrob Agents Chemother 43(1):1–11
De Lucca AJ, Walsh TJ (2000) Antifungal peptides: origin, activity, and therapeutic potential. Revista iberoamericana de micologia 17(4):116–120
De Smet K, Contreras R (2005) Human antimicrobial peptides: defensins, cathelicidins and histatins. Biotechnol Lett 27(18):1337–1347
de Waal A, Gomes AV et al (1991) Magainins affect respiratory control, membrane potential and motility of hamster spermatozoa. FEBS Lett 293(1–2):219–223
del Castillo FJ, del Castillo I et al (2001) Construction and characterization of mutations at codon 751 of the Escherichia coli gyrB gene that confer resistance to the antimicrobial peptide microcin B17 and alter the activity of DNA gyrase. J Bacteriol 183(6):2137–2140
Destoumieux D, Bulet P et al (1999) Recombinant expression and range of activity of penaeidins, antimicrobial peptides from penaeid shrimp. Eur J Biochem 266(2):335–346
Destoumieux D, Munoz M et al (2000) Penaeidins, a family of antimicrobial peptides from penaeid shrimp (Crustacea, Decapoda). Cell Mol Life Sci CMLS 57(8–9):1260–1271
Diamond G, Jones DE et al (1993) Airway epithelial cells are the site of expression of a mammalian antimicrobial peptide gene. Proc Natl Acad Sci 90(10):4596–4600
Dimarcq JL, Bulet P et al (1998) Cysteine-rich antimicrobial peptides in invertebrates. Pept Sci 47(6):465–477
Divyashree M, Mani MK et al (2020) Clinical applications of antimicrobial peptides (AMPs): where do we stand now? Protein Pept Lett 27(2):120–134
Dong Y, Aguilar R et al (2006) Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathog 2(6):e52
Dong Y, Taylor HE et al (2006) AgDscam, a hypervariable immunoglobulin domain-containing receptor of the Anopheles gambiae innate immune system. PLoS Biol 4(7):e229
Dubos RJ (1939a) Studies on a bactericidal agent extracted from a soil bacillus: I. Preparation of the agent. Its activity in vitro. J Exp Med 70(1):1
Dubos RJ (1939b) Studies on a bactericidal agent extracted from a soil bacillus: II. Protective effect of the bactericidal agent against experimental Pneumococcus infections in mice. J Exp Med 70(1):11–17
Dubos RJ, Hotchkiss RD (1941) The production of bactericidal substances by aerobic sporulating bacilli. J Exp Med 73(5):629–640
Dufour D, Leung V et al (2010) Bacterial biofilm: structure, function, and antimicrobial resistance. Endod Top 22(1):2–16
Ebenhan T, Gheysens O et al (2014) Antimicrobial peptides: their role as infection-selective tracers for molecular imaging. BioMed Res Int 2014:15
Edelstein MC, Gretz JE et al (1991) Studies on the in vitro spermicidal activity of synthetic magainins. Fertil Steril 55(3):647–649
Ehrenstein G, Lecar H (1977) Electrically gated ionic channels in lipid bilayers. Q Rev Biophys 10(1):1–34
Ehret-Sabatier L, Loew D et al (1996) Characterization of novel cysteine-rich antimicrobial peptides from scorpion blood. J Biol Chem 271(47):29537–29544
Eisenberg D (1984) Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem 53(1):595–623
Elsbach P (2003) What is the real role of antimicrobial polypeptides that can mediate several other inflammatory responses? J Clin Investig 111(11):1643–1645
Erak M, Bellmann-Sickert K et al (2018) Peptide chemistry toolbox–Transforming natural peptides into peptide therapeutics. Bioorg Med Chem 26(10):2759–2765
Falla TJ, Karunaratne DN et al (1996) Mode of action of the antimicrobial peptide indolicidin. J Biol Chem 271(32):19298–19303
Fan L, Sun J et al (2016) DRAMP: a comprehensive data repository of antimicrobial peptides. Sci Rep 6:24482
Farcas A, Buimaga-Iarinca L et al (2020) Design of novel antimicrobial peptides in a multi-stage in silico approach. Biophys J 118(3):384
Fearon DT, Locksley RM (1996) The instructive role of innate immunity in the acquired immune response. Science 272(5258):50–54
Fehlbaum P, Bulet P et al (1996) Structure-activity analysis of thanatin, a 21-residue inducible insect defense peptide with sequence homology to frog skin antimicrobial peptides. Proc Natl Acad Sci 93(3):1221–1225
Fernández-Vidal M, Jayasinghe S et al (2007) Folding amphipathic helices into membranes: amphiphilicity trumps hydrophobicity. J Mol Biol 370(3):459–470
Fjell CD, Hancock RE et al (2007) AMPer: a database and an automated discovery tool for antimicrobial peptides. Bioinformatics 23(9):1148–1155
Fjell CD, Jenssen H et al (2008) Identification of novel host defense peptides and the absence of α-defensins in the bovine genome. Proteins Struct Funct Bioinf 73(2):420–430
Fjell CD, Hiss JA et al (2012a) Designing antimicrobial peptides: form follows function. Nat Rev Drug Discov 11(1):37
Fjell CD, Hiss JA et al (2012b) Designing antimicrobial peptides: form follows function. Nat Rev Drug Discov 11(1):37–51
Fosgerau K, Hoffmann T (2015) Peptide therapeutics: current status and future directions. Drug Discov Today 20(1):122–128
Fox JL (2013) Antimicrobial peptides stage a comeback. Nature Publishing Group, Berlin
Friedrich C, Scott MG et al (1999) Salt-resistant alpha-helical cationic antimicrobial peptides. Antimicrob Agents Chemother 43(7):1542–1548
Fukumoto K, Nagaoka I et al (2005) Effect of antibacterial cathelicidin peptide CAP18/LL-37 on sepsis in neonatal rats. Pediatr Surg Int 21(1):20–24
Furniss RCD, Kostrzewa M et al (2020) The clue is in the lipid A: rapid detection of colistin resistance. PLoS Pathog 16(4):e1008331
Gajdács M (2019) The concept of an ideal antibiotic: implications for drug design. Molecules 24(5):892
Gajdács M (2020) Carbapenem-resistant but cephalosporin-susceptible pseudomonas aeruginosa in urinary tract infections opportunity for colistin sparing. Antibiotics 9(4):153
Gajdács M, Albericio F (2019) Antibiotic resistance: from the bench to patients. Multidisciplinary Digital Publishing Institute, Basel
Gajdács M, Urbán E (2019) Comparative epidemiology and resistance trends of proteae in urinary tract infections of inpatients and outpatients: a 10-year retrospective study. Antibiotics 8(3):91
Ganguly D, Chamilos G et al (2009) Self-RNA–antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J Exp Med 206(9):1983–1994
Ganz T, Selsted ME et al (1985) Defensins. Natural peptide antibiotics of human neutrophils. J Clin Investig 76(4):1427–1435
García-Olmedo F, Molina A et al (1998) Plant defense peptides. Pept Sci 47(6):479–491
Gesell J, Zasloff M et al (1997) Two-dimensional 1H NMR experiments show that the 23-residue magainin antibiotic peptide is an α-helix in dodecylphosphocholine micelles, sodium dodecylsulfate micelles, and trifluoroethanol/water solution. J Biomol NMR 9(2):127–135
Giuliani A, Pirri G et al (2007) Antimicrobial peptides: an overview of a promising class of therapeutics. Open Life Sci 2(1):1–33
Gombart AF, Saito T et al (2009) Exaptation of an ancient Alu short interspersed element provides a highly conserved vitamin D-mediated innate immune response in humans and primates. BMC Genomics 10(1):321
Gorbach SL (2001) Antimicrobial use in animal feed—Time to stop. Mass Medical Soc, Waltham
Guina T, Eugene CY et al (2000) A PhoP-regulated outer membrane protease of Salmonella enterica serovar Typhimurium promotes resistance to alpha-helical antimicrobial peptides. J Bacteriol 182(14):4077–4086
Gunn JS (2001) Bacterial modification of LPS and resistance to antimicrobial peptides. J Endotoxin Res 7(1):57–62
Guo L, Lim KB et al (1998) Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell 95(2):189–198
Guthmiller JM, Vargas KG et al (2001) Susceptibilities of oral bacteria and yeast to mammalian cathelicidins. Antimicrob Agents Chemother 45(11):3216–3219
Hale JD, Hancock RE (2007) Alternative mechanisms of action of cationic antimicrobial peptides on bacteria. Expert Rev Anti-infect Ther 5(6):951–959
Hammami R, Fliss I (2010) Current trends in antimicrobial agent research: chemo-and bioinformatics approaches. Drug Discov Today 15(13–14):540–546
Hancock RE (1997) Peptide antibiotics. Lancet 349(9049):418–422
Hancock RE (2000) Cationic antimicrobial peptides: towards clinical applications. Expert Opin investig Drugs 9(8):1723–1729
Hancock RE, Diamond G (2000) The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol 8(9):402–410
Hancock RE, Lehrer R (1998) Cationic peptides: a new source of antibiotics. Trends Biotechnol 16(2):82–88
Hancock RE, Scott MG (2000) The role of antimicrobial peptides in animal defenses. Proc Natl Acad Sci 97(16):8856–8861
Haney EF, Nazmi K et al (2009) Novel lactoferrampin antimicrobial peptides derived from human lactoferrin. Biochimie 91(1):141–154
Henderson JC, Fage CD et al (2014) Antimicrobial peptide resistance of Vibrio cholerae results from an LPS modification pathway related to nonribosomal peptide synthetases. ACS Chem Biol 9(10):2382–2392
Hilpert K, McLeod B et al (2010) Short cationic antimicrobial peptides interact with ATP. Antimicrob Agents Chemother 54(10):4480–4483
Hirsch JG (1956) Phagocytin: a bactericidal substance from polymorphonuclear leucocytes. J Exp Med 103(5):589–611
Hotchkiss RD, Dubos RJ (1940) Fractionation of the bactericidal agent from cultures of a soil bacillus. J Biol Chem 132(2):791–792
Huang Y, Huang J et al (2010) Alpha-helical cationic antimicrobial peptides: relationships of structure and function. Protein Cell 1(2):143–152
Hultmark D (2003) Drosophila immunity: paths and patterns. Curr Opin Immunol 15(1):12–19
Hultmark D, Steiner H et al (1980) Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur J Biochem 106(1):7–16
Imler J-L, Bulet P (2005) Antimicrobial peptides in Drosophila: structures, activities and gene regulation. In: Kabelitz D, Schröder JM (eds) Mechanisms of epithelial defense. Karger Publishers, Basel, pp 1–21
Jarosz J (1997) Identification of immune inhibitor from Pseudomonas aeruginosa of inducible cell-free antibacterial activity in insects. Cytobios 89(357):73–80
Jarosz J, Gliński Z (1990) Selective inhibition of cecropin-like activity of insect immune blood by protease from American foulbrood scales. J Invertebr Pathol 56(2):143–149
Jenssen H, Hamill P et al (2006) Peptide antimicrobial agents. Clin Microbiol Rev 19(3):491–511
Jiang Z, Vasil AI et al (2008) Effects of net charge and the number of positively charged residues on the biological activity of amphipathic α-helical cationic antimicrobial peptides. Pept Sci 90(3):369–383
Johansson J, Gudmundsson GH et al (1998) Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J Biol Chem 273(6):3718–3724
Johnstone SA, Gelmon K et al (2000) In vitro characterization of the anticancer activity of membrane-active cationic peptides. I. Peptide-mediated cytotoxicity and peptide-enhanced cytotoxic activity of doxorubicin against wild-type and p-glycoprotein over-expressing tumor cell lines. Anti-Cancer Drug Des 15(2):151–160
Jones AT (2007) Macropinocytosis: searching for an endocytic identity and role in the uptake of cell penetrating peptides. J Cell Mol Med 11(4):670–684
Juretic D, Vukicevic D et al (2009) Computational design of highly selective antimicrobial peptides. J Chem Inf Model 49(12):2873–2882
Jusko M, Potempa J et al (2012) A metalloproteinase karilysin present in the majority of Tannerella forsythia isolates inhibits all pathways of the complement system. J Immunol 188(5):2338–2349
Kang X, Dong F et al (2019) DRAMP 2.0, an updated data repository of antimicrobial peptides. Sci Data 6(1):1–10
Kaspar AA, Reichert JM (2013) Future directions for peptide therapeutics development. Drug Discov Today 18(17–18):807–817
Kavanagh K, Dowd S (2004) Histatins: antimicrobial peptides with therapeutic potential. J Pharm Pharmacol 56(3):285–289
Kawabata S-I, Nagayama R et al (1996) Tachycitin, a small granular component in horseshoe crab hemocytes, is an antimicrobial protein with chitin-binding activity. J Biochem 120(6):1253–1260
Kim DH, Lee DG et al (2001) Internalization of tenecin 3 by a fungal cellular process is essential for its fungicidal effect on Candida albicans. Eur J Biochem 268(16):4449–4458
Koczulla R, Von Degenfeld G et al (2003) An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Investig 111(11):1665–1672
Koo JC, Lee B et al (2004) Pn-AMP1, a plant defense protein, induces actin depolarization in yeasts. Plant Cell Physiol 45(11):1669–1680
Kowalczyk R, Harris PW et al (2017) Peptide lipidation–a synthetic strategy to afford peptide based therapeutics. In: Care A, Bergquist PL (eds) Sunna A. Peptides and peptide-based biomaterials and their biomedical applications, Springer, New York, pp 185–227
Kragol G, Lovas S et al (2001) The antibacterial peptide pyrrhocoricin inhibits the ATPase actions of DnaK and prevents chaperone-assisted protein folding. Biochemistry 40(10):3016–3026
Kristian SA, Datta V et al (2005) D-alanylation of teichoic acids promotes group a streptococcus antimicrobial peptide resistance, neutrophil survival, and epithelial cell invasion. J Bacteriol 187(19):6719–6725
Kumar P, Kizhakkedathu JN et al (2018) Antimicrobial peptides: diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules 8(1):4
Kustanovich I, Shalev DE et al (2002) Structural requirements for potent versus selective cytotoxicity for antimicrobial dermaseptin S4 derivatives. J Biol Chem 277(19):16941–16951
Lai X-Z, Feng Y et al (2008) Ceragenins: cholic acid-based mimics of antimicrobial peptides. Acc Chem Res 41(10):1233–1240
Lai Y, Gallo RL (2009) AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol 30(3):131–141
Langer T, Hoffmann R et al (2009) Hit finding: towards ‘smarter’approaches. Curr Opin Pharmacol 9(5):589–593
Lee YT, Kim DH et al (1999) Structural characteristics of tenecin 3, an insect antifungal protein. IUBMB Life 47(3):369–376
Lee DG, Kim HN et al (2002) Design of novel analogue peptides with potent antibiotic activity based on the antimicrobial peptide, HP (2–20), derived from N-terminus of Helicobacter pylori ribosomal protein L1. Biochim Biophys Acta 1598(1–2):185–194
Lee DG, Kim PI et al (2002) Design of novel peptide analogs with potent fungicidal activity, based on PMAP-23 antimicrobial peptide isolated from porcine myeloid. Biochem Biophys Res Commun 293(1):231–238
Lee DG, Kim HK et al (2003) Fungicidal effect of indolicidin and its interaction with phospholipid membranes. Biochem Biophys Res Commun 305(2):305–310
Lehrer RI, Ganz T (1999) Antimicrobial peptides in mammalian and insect host defence. Curr Opin Immunol 11(1):23–27
Lehrer RI, Daher K et al (1985) Direct inactivation of viruses by MCP-1 and MCP-2, natural peptide antibiotics from rabbit leukocytes. J Virol 54(2):467–472
Lehrer RI, Lichtenstein AK et al (1993) Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol 11(1):105–128
Leippe M (1999) Antimicrobial and cytolytic polypeptides of amoeboid protozoa-effector molecules of primitive phagocytes. Dev Comp Immunol 23(4–5):267–279
Lewis LA, Choudhury B et al (2009) Phosphoethanolamine substitution of lipid A and resistance of Neisseria gonorrhoeae to cationic antimicrobial peptides and complement-mediated killing by normal human serum. Infect Immun 77(3):1112–1120
Lichtenstein A, Ganz T et al (1986) In vitro tumor cell cytolysis mediated by peptide defensins of human and rabbit granulocytes. Blood 68(6):1407–1410
Macedo Timmers LFS, Pauli I et al (2008) Drug-binding databases. Curr Drug Targets 9(12):1092–1099
Madani F, Lindberg S et al (2011) Mechanisms of cellular uptake of cell-penetrating peptides. J Biophys 2011:10
Mahlapuu M, Håkansson J et al (2016) Antimicrobial peptides: an emerging category of therapeutic agents. Front Cell Infect Microbiol 6:194
Malanovic N, Lohner K (2016) Antimicrobial peptides targeting gram-positive bacteria. Pharmaceuticals 9(3):59
Mant CT, Chen Y et al (2003) Temperature profiling of polypeptides in reversed-phase liquid chromatography: I. Monitoring of dimerization and unfolding of amphipathic α-helical peptides. J Chromatogr A 1009(1–2):29–43
Marcos JF, Beachy RN et al (1995) Inhibition of a plant virus infection by analogs of melittin. Proc Natl Acad Sci 92(26):12466–12469
Maria-Neto S, de Almeida KC et al (2015) Understanding bacterial resistance to antimicrobial peptides: from the surface to deep inside. Biochim Biophys Acta 1848(11):3078–3088
Marr AK, Gooderham WJ et al (2006) Antibacterial peptides for therapeutic use: obstacles and realistic outlook. Curr Opin Pharmacol 6(5):468–472
Marwick C (1999) Animal feed antibiotic use raises drug resistance fear. JAMA 282(2):120–122
Matanic VCA, Castilla V (2004) Antiviral activity of antimicrobial cationic peptides against Junin virus and herpes simplex virus. Int J Antimicrob Agents 23(4):382–389
Matsuzaki K (1998) Magainins as paradigm for the mode of action of pore forming polypeptides. Biochim Biophys Acta 1376(3):391–400
Matsuzaki K (1999) Why and how are peptide–lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim Biophys Acta 1462(1–2):1–10
Matsuzaki K (2009) Control of cell selectivity of antimicrobial peptides. Biochim Biophys Acta 1788(8):1687–1692
Matsuzaki K, Sugishita K et al (1995) Molecular basis for membrane selectivity of an antimicrobial peptide, magainin 2. Biochemistry 34(10):3423–3429
Matsuzaki K, Murase O et al (1996) An antimicrobial peptide, magainin 2, induced rapid flip-flop of phospholipids coupled with pore formation and peptide translocation. Biochemistry 35(35):11361–11368
Mayor S, Pagano RE (2007) Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol 8(8):603
Mishra NN, Bayer AS (2013) Correlation of cell membrane lipid profiles with daptomycin resistance in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 57(2):1082–1085
Moradi SV, Hussein WM et al (2016) Glycosylation, an effective synthetic strategy to improve the bioavailability of therapeutic peptides. Chem Sci 7(4):2492–2500
Murakami T, Niwa M et al (1991) Direct virus inactivation of tachyplesin I and its isopeptides from horseshoe crab hemocytes. Chemotherapy 37(5):327–334
Mygind PH, Fischer RL et al (2005) Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature 437(7061):975
Nagaoka I, Kuwahara-Arai K et al (2005) Augmentation of the bactericidal activities of human cathelicidin CAP18/LL-37-derived antimicrobial peptides by amino acid substitutions. Inflamm Res 54(2):66–73
Nakamura T, Furunaka H et al (1988) Tachyplesin, a class of antimicrobial peptide from the hemocytes of the horseshoe crab (Tachypleus tridentatus). Isolation and chemical structure. J Biol Chem 263(32):16709–16713
Nguyen LT, Haney EF et al (2011) The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol 29(9):464–472
Nicolas P (2009) Multifunctional host defense peptides: intracellular-targeting antimicrobial peptides. FEBS J 276(22):6483–6496
Niu Y, Wang RE et al (2012) Recent development of small antimicrobial peptidomimetics. Future Med Chem 4(14):1853–1862
Oren Z, Shai Y (1996) A class of highly potent antibacterial peptides derived from pardaxin, a pore-forming peptide isolated from Moses sole fish Pardachirus marmoratus. Eur J Biochem 237(1):303–310
Otvos L et al (2000) Interaction between heat shock proteins and antimicrobial peptides. Biochemistry 39(46):14150–14159
Pace CN, Scholtz JM (1998) A helix propensity scale based on experimental studies of peptides and proteins. Biophys J 75(1):422–427
Panyutich A, Ganz T (1991) Activated oe-macroglobulin Is a principal defensin-binding protein. Am J Respir Cell Mol BioI Vol 5:101–106
Papo N, Shai Y (2003) Can we predict biological activity of antimicrobial peptides from their interactions with model phospholipid membranes? Peptides 24(11):1693–1703
Papo N, Oren Z et al (2002) The consequence of sequence alteration of an amphipathic α-helical antimicrobial peptide and its diastereomers. J Biol Chem 277(37):33913–33921
Parachin NS, Franco OL (2014) New edge of antibiotic development: antimicrobial peptides and corresponding resistance. Front Microbiol 5:147
Park Y, Jang SH et al (2004) Antinematodal effect of antimicrobial peptide, PMAP-23, isolated from porcine myeloid against Caenorhabditis elegans. J Pept Sci 10(5):304–311
Park Y, Park SC et al (2007) Structure-activity relationship of HP (2–20) analog peptide: enhanced antimicrobial activity by N‐terminal random coil region deletion. Pept Sci 88(2):199–207
Pasupuleti M, Schmidtchen A et al (2012) Antimicrobial peptides: key components of the innate immune system. Crit Rev Biotechnol 32(2):143–171
Patrzykat A, Friedrich CL et al (2002) Sublethal concentrations of pleurocidin-derived antimicrobial peptides inhibit macromolecular synthesis in Escherichia coli. Antimicrob Agents Chemother 46(3):605–614
Peck-Miller KA et al (1994) Structure‐activity analysis of the antitumor and hemolytic properties of the amphiphilic α‐helical peptide, C18G. Int J Pept Protein Res 44(2):143–151
Peschel A, Sahl H-G (2006) The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat Rev Microbiol 4(7):529
Pouny Y, Rapaport D et al (1992) Interaction of antimicrobial dermaseptin and its fluorescently labeled analogs with phospholipid membranes. Biochemistry 31(49):12416–12423
Powers J-PS, Hancock RE (2003) The relationship between peptide structure and antibacterial activity. Peptides 24(11):1681–1691
Raetz CR, Whitfield C (2002) Lipopolysaccharide endotoxins. Annu Rev Biochem 71(1):635–700
Reddy K, Yedery R et al (2004) Antimicrobial peptides: premises and promises. Int J Antimicrob Agents 24(6):536–547
Resnick NM, Maloy WL et al (1991) A novel endopeptidase from Xenopus that recognizes α-helical secondary structure. Cell 66(3):541–554
Rinanda T (2019) In Silico Studies in Antimicrobial Peptides Design and Development. IOP Conference Series: Earth and Environmental Science, IOP Publishing
Robinson WE Jr, McDougall B et al (1998) Anti-HIV‐1 activity of indolicidin, an antimicrobial peptide from neutrophils. J Leukoc Biol 63(1):94–100
Rollins-Smith LA, Carey C et al (2002) Activity of antimicrobial skin peptides from ranid frogs against Batrachochytrium dendrobatidis, the chytrid fungus associated with global amphibian declines. Dev Comp Immunol 26(5):471–479
Rotem S, Mor A (2009) Antimicrobial peptide mimics for improved therapeutic properties. Biochim Biophys Acta 1788(8):1582–1592
Rothstein DM, Spacciapoli P et al (2001) Anticandida activity is retained in P-113, a 12-amino-acid fragment of histatin 5. Antimicrob Agents Chemother 45(5):1367–1373
Saito T, Kawabata S-I et al (1995) A novel big defensin identified in horseshoe crab hemocytes: isolation, amino acid sequence, and antibacterial activity. J Biochem 117(5):1131–1137
Sambri V, Marangoni A et al (2002) Comparative in vitro activity of five cathelicidin-derived synthetic peptides against Leptospira, Borrelia and Treponema pallidum. J Antimicrob Chemother 50(6):895–902
Sawa T, Kurahashi K et al (1998) Evaluation of antimicrobial and lipopolysaccharide-neutralizing effects of a synthetic CAP18 fragment against Pseudomonas aeruginosa in a mouse model. Antimicrob Agents Chemother 42(12):3269–3275
Sawicki W, Mystkowska ET (1999) Contraceptive potential of peptide antibiotics. Lancet 353(9151):464–465
Schauber J, Gallo RL (2008) Antimicrobial peptides and the skin immune defense system. J Allergy Clin Immunol 122(2):261–266
Selsted ME, Novotny MJ et al (1992) Indolicidin, a novel bactericidal tridecapeptide amide from neutrophils. J Biol Chem 267(7):4292–4295
Shafer W, Qu X-D et al (1998) Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc Natl Acad Sci 95(4):1829–1833
Shai Y (2002) Mode of action of membrane active antimicrobial peptides. Pept Sci 66(4):236–248
Shi J, Ganz T (1998) The role of protegrins and other elastase-activated polypeptides in the bactericidal properties of porcine inflammatory fluids. Infect Immun 66(8):3611–3617
Shimazaki K, Tazume T et al (1998) Properties of a heparin-binding peptide derived from bovine lactoferrin. J Dairy Sci 81(11):2841–2849
Skerlavaj B, Gennaro R et al (1996) Biological characterization of two novel cathelicidin-derived peptides and identification of structural requirements for their antimicrobial and cell lytic activities. J Biol Chem 271(45):28375–28381
Skerlavaj B, Benincasa M et al (1999) SMAP-29: a potent antibacterial and antifungal peptide from sheep leukocytes. FEBS Lett 463(1–2):58–62
Spellberg B (2014) The future of antibiotics. Crit Care 18(3):228
Steiner H, Hultmark D et al (1981) Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature 292(5820):246
Subbalakshmi C, Sitaram N (1998) Mechanism of antimicrobial action of indolicidin. FEMS Microbiol Lett 160(1):91–96
Subbalakshmi C, Nagaraj R et al (1999) Biological activities of C-terminal 15‐residue synthetic fragment of melittin: design of an analog with improved antibacterial activity. FEBS Lett 448(1):62–66
Teixeira V, Feio MJ et al (2012) Role of lipids in the interaction of antimicrobial peptides with membranes. Prog Lipid Res 51(2):149–177
Tossi A, Sandri L et al (2000) Amphipathic, α-helical antimicrobial peptides. Pept Sci 55(1):4–30
Tsai H, Bobek L (1998) Human salivary histatins: promising anti-fungal therapeutic agents. Crit Rev Oral Biol Med 9(4):480–497
Uhlig T, Kyprianou T et al (2014) The emergence of peptides in the pharmaceutical business: from exploration to exploitation. EuPA Open Proteom 4:58–69
Van Epps HL (2006) René Dubos: unearthing antibiotics. J Exp Med 203(2):259–259
Vivero-Escoto JL, Slowing II et al (2010) Mesoporous silica nanoparticles for intracellular controlled drug delivery. Small 6(18):1952–1967
Vizioli J, Salzet M (2002) Antimicrobial peptides from animals: focus on invertebrates. Trends Pharmacol Sci 23(11):494–496
Vizioli J, Bulet P et al (2001) Gambicin: a novel immune responsive antimicrobial peptide from the malaria vector Anopheles gambiae. Proc Natl Acad Sci 98(22):12630–12635
Wachinger M, Saermark T et al (1992) Influence of amphipathic peptides on the HIV-1 production in persistently infected T lymphoma cells. FEBS Lett 309(3):235–241
Wachinger M, Kleinschmidt A et al (1998) Antimicrobial peptides melittin and cecropin inhibit replication of human immunodeficiency virus 1 by suppressing viral gene expression. J Gen Virol 79(4):731–740
Wang G, Li X et al (2008) APD2: the updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res 37(suppl_1):D933–D937
Wang J, Dou X et al (2019) Antimicrobial peptides: promising alternatives in the post feeding antibiotic era. Med Res Rev 39(3):831–859
Watkins RR, Bonomo RA (2016) Overview: global and local impact of antibiotic resistance. Infect Dis Clin 30(2):313–322
Westerhoff HV, Juretić D et al (1989) Magainins and the disruption of membrane-linked free-energy transduction. Proc Natl Acad Sci 86(17):6597–6601
Wieprecht T, Dathe M et al (1997) Peptide hydrophobicity controls the activity and selectivity of magainin 2 amide in interaction with membranes. Biochemistry 36(20):6124–6132
Willey JM, van der Donk WA (2007) Lantibiotics: peptides of diverse structure and function. Annu Rev Microbiol 61:477–501
Wu M, Maier E et al (1999) Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry 38(22):7235–7242
Yang D, Biragyn A et al (2002) Mammalian defensins in immunity: more than just microbicidal. Trends Immunol 23(6):291–296
Yasin B, Pang M et al (2000) Evaluation of the inactivation of infectious herpes simplex virus by host-defense peptides. Eur J Clin Microbiol Infect Dis 19(3):187–194
Yeaman MR, Yount NY (2003) Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev 55(1):27–55
Yeaman MR, Bayer AS et al (1998) Platelet microbicidal proteins and neutrophil defensin disrupt the Staphylococcus aureus cytoplasmic membrane by distinct mechanisms of action. J Clin Investig 101(1):178–187
Yeung AT, Gellatly SL et al (2011) Multifunctional cationic host defence peptides and their clinical applications. Cell Mol Life Sci 68(13):2161
Yount NY, Yeaman MR (2005) Immunocontinuum: perspectives in antimicrobial peptide mechanisms of action and resistance. Protein Pept Lett 12(1):49–67
Zanetti M, Litteri L et al (1990) Bactenecins, defense polypeptides of bovine neutrophils, are generated from precursor molecules stored in the large granules. J Cell Biol 111(4):1363–1371
Zanetti M, Gennaro R et al (1997) The cathelicidin family of antimicrobial peptide precursors: a component of the oxygen-independent defense mechanisms of neutrophils A. Ann N Y Acad Sci 832(1):147–162
Zasloff M (1987) Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci 84(15):5449–5453
Zasloff M (1992) Antibiotic peptides as mediators of innate immunity. Curr Opin Immunol 4(1):3–7
Zasloff M (2002a) Antimicrobial peptides of multicellular organisms. Nature 415(6870):389
Zasloff M (2002b) Innate immunity, antimicrobial peptides, and protection of the oral cavity. Lancet 360(9340):1116–1117
Zelezetsky I, Pacor S et al (2005) Controlled alteration of the shape and conformational stability of α-helical cell-lytic peptides: effect on mode of action and cell specificity. Biochem J 390(1):177–188
Zeya H, Spitznagel J (1966) Cationic proteins of polymorphonuclear leukocyte lysosomes I. Resolution of antibacterial and enzymatic activities. J Bacteriol 91(2):750–754
Zhang L, Benz R et al (1999) Influence of proline residues on the antibacterial and synergistic activities of α-helical peptides. Biochemistry 38(25):8102–8111
Zhang L, Rozek A et al (2001) Interaction of cationic antimicrobial peptides with model membranes. J Biol Chem 276(38):35714–35722
Zhang L, Yu W et al (2002) Contribution of human α-defensin 1, 2, and 3 to the anti-HIV-1 activity of CD8 antiviral factor. Science 298(5595):995–1000
Zhang T, Muraih JK et al (2014) Cardiolipin prevents membrane translocation and permeabilization by daptomycin. J Biol Chem 289(17):11584–11591
Acknowledgements
The authors are grateful to Assam University, Silchar, Assam for providing necessary facilities to carry out this research work.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest in the manuscript.
Informed Consent
Approved.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Borah, A., Deb, B. & Chakraborty, S. A Crosstalk on Antimicrobial Peptides. Int J Pept Res Ther 27, 229–244 (2021). https://doi.org/10.1007/s10989-020-10075-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-020-10075-x