Abstract
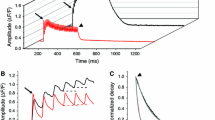
[Ca2+] transients inside the sarcoplasmic reticulum (SR) were recorded in frog skeletal muscle twitch fibers under voltage clamp using the low affinity indicator Mag Fluo 4 (loaded in its AM form) with the purpose of studying the effect on Ca2+ release of extrinsic Ca2+ buffers (i.e. BAPTA) added at high concentration to the myoplasm. When the extrinsic Ca2+ buffer is added to the myoplasm, part of the released Ca2+ binds to it, reducing the Ca2+ signal reported by a myoplasmic indicator. This, in turn, hinders the quantification of the amount of Ca2+ released. Monitoring release by measuring [Ca2+] inside the SR avoids this problem. The application of extrinsic buffers at high concentration reduced the resting [Ca2+] in the SR ([Ca2+]SR) continuously from a starting value close to 400 μM reaching the range of 100 μM in about half an hour. The effect of reducing resting [Ca2+]SR on the Ca2+ permeability of the SR activated by voltage clamp depolarization to 0 mV was studied in cells where the myoplasmic [Ca2+] ([Ca2+]myo) transients were simultaneously recorded with Rhod2. The Ca2+ release flux was calculated from [Ca2+]myo and divided by [Ca2+]SR to obtain the permeability. Peak permeability was significantly reduced, from 0.026 ± 0.005 ms−1 at resting [Ca2+]SR = 372 ± 5 μM to 0.021 ± 0.004 ms−1 at resting [Ca2+]SR = 120 ± 16 μM (n = 4, p = 0.03). The time averaged permeability was not significantly changed (0.009 ± 0.003 and 0.010 ± 0.003 ms−1, at the higher and lower [Ca2+]SR respectively). Once the cells were equilibrated with the high buffer intracellular solution, the change in [Ca2+]SR (Δ[Ca2+]SR) in response to voltage clamp depolarization (0 mV, 200 ms) in 20 mM BAPTA was significantly lower (Δ[Ca2+]SR = 30.2 ± 3.5 μM from resting [Ca2+]SR = 88.8 ± 13.6 μM, n = 5) than in 40 mM EGTA (Δ[Ca2+]SR = 72.2 ± 10.4 μM from resting [Ca2+]SR = 98.2 ± 15.6 μM, n = 4) suggesting that a Ca2+ activated component of release was suppressed by BAPTA.








Similar content being viewed by others
References
Beard NA, Laver DR, Dulhunty AF (2004) Calsequestrin and the calcium release channel of skeletal and cardiac muscle. Prog Biophys Mol Biol 85(1):33–69
Block BA, Imagawa T, Campbell KP, Franzini-Armstrong C (1988) Structural evidences for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol 107:2587–2600
Brum G, Ríos E, Stefani E (1988a) Effects of extracellular calcium on calcium movements of excitation-contraction coupling in frog skeletal muscle fibres. J Physiol 398:441–473
Brum G, Fitts R, Pizarro G, Ríos E (1988b) Voltage sensors of the frog skeletal muscle membrane require calcium to function in excitation-contraction coupling. J Physiol 398:475–505
Canato M, Scorzeto M, Giacomello M, Protasi F, Reggiani C, Stienen GJ (2010) Massive alterations of sarcoplasmic reticulum free calcium in skeletal muscle fibers lacking calsequestrin revealed by a genetically encoded probe. Proc Natl Acad Sci USA 107:22326–22331
Cheng H, Lederer WJ (2008) Calcium sparks. Physiol Rev 88(4):1491–1545
Csernoch L, Jacquemond V, Schneider MF (1993) Microinjection of strong calcium buffers suppresses the peak of calcium release during depolarization in frog skeletal muscle fibers. J Gen Physiol 101(2):297–333
Cukierman S, Yellen G, Miller C (1985) The K+ channel of sarcoplasmic reticulum. A new look at Cs + block. Biophys J 48(3):477–484
De Armas R, González S, Brum G, Pizarro G (1998) Effects of 2,3-butanedione monoxime on excitation-contraction coupling in frog twitch fibres. J Mus Res Cell Motil 19:961–977
Donoso P, Prieto H, Hidalgo C (1995) Luminal calcium regulates calcium release in triads isolated from frog and rabbit skeletal muscle. Biophys J 68:507–515
Endo M (2009) Calcium-induced calcium release in skeletal muscle. Physiol Rev 89(4):1153–1176
Endo M, Tanaka M, Ogawa Y (1970) Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature 228:34–36
Felder E, Franzini-Armstrong C (2002) Type 3 ryanodine receptors of skeletal muscle are segregated in a parajunctional position. Proc Natl Acad Sci USA 99(3):1695–1700
Fénelon K, Pape PC (2002) Recruitment of Ca2+ release channels by calcium-induced Ca2+ release does not appear to occur in isolated Ca2+ release sites. J Physiol 544(3):777–791
Fénelon K, Lamboley CR, Carrier N, Pape PC (2012) Calcium buffering properties of sarcoplasmic reticulum and calcium-induced Ca2+ release during the quasi-steady level of release in twitch fibers from frog skeletal muscle. J Gen Physiol 140(4):403–419
Fill M, Copello J (2002)). Ryanodine receptors calcium channels. Physiol Rev 82:893–922
Gillespie D, Fill M (2008) Intracellular calcium release channels mediate their own countercurrent: the ryanodine receptor case study. Biophys J 95(8):3706–3714
González A, Ríos E (1993) Perchlorate enhances transmission in skeletal muscle excitation contraction coupling. J Gen Physiol 102:373 421
Györke S, Györke I, Lukyanenko V, Terentyev D, Viatchenko-Karpinski S, Wiesner TF (2002) Regulation of sarcoplasmic reticulum calcium release by luminal calcium in cardiac muscle. Front Biosci 1(7):1454–1463
Harafuji H, Ogawa Y (1980) Re-examination of the apparent binding constant of ethylene glycol bis(beta-aminoethyl ether)-N,N,N′,N′-tetraacetic acid with calcium around neutral pH. J Biochem 87(5):1305–1312
Hollingworth S, Gee KR, Baylor SM (2009) Low-affinity Ca indicators compared in measurements of skeletal muscle Ca2+ transients. Biophys J 97(7):1864–1872
Jacquemond V, Csernoch L, Klein MG, Schneider MF (1991) Voltage-gated and calcium-gated calcium release during depolarization of skeletal muscle fibers. Biophys J 60(4):867–873
Jong DS, Pape PC, Chandler WK, Baylor SM (1993) Reduction of calcium inactivation of sarcoplasmic reticulum calcium release by fura-2 in voltage-clamped cut twitch fibers from frog muscle. J Gen Physiol 102:333–370
Jong DS, Pape PC, Baylor SM, Chandler WK (1995) Calcium inactivation of calcium release in frog cut muscle fibers that contain millimolar EGTA or Fura-2. J Gen Physiol 106:337–388
Kabbara AA, Allen DG (2001) The use of the indicator fluo-5N to measure sarcoplasmic reticulum calcium in single muscle fibres of the cane toad. J Physiol 534:87–97
Kashiyama T, Murayama T, Suzuki E, Allen PD, Ogawa Y (2010) Frog alpha- and beta-ryanodine receptors provide distinct intracellular Ca2 + signals in a myogenic cell line. PLoS One 12;5(7):e11526
Kettlun C, González A, Ríos E, Fill M (2003) Unitary Ca2 + current through mammalian cardiac and amphibian skeletal muscle ryanodine receptor channels under near-physiological ionic conditions. J Gen Physiol 122(4):407–417
Kits KS, de Vlieger TA, Kooi BW, Mansvelder HD (1999) Diffusion barriers limit the effect of mobile calcium buffers on exocytosis of large dense cored vesicles. Biophys J. 76(3):1693–1705
Kovacs L, Ríos E, Schneider MF (1983) Measurement and modification of free calcium transients in frog skeletal muscle fibres by metallochromic indicator dye. J Physiol 343:161–196
Labarca PP, Miller C (1981) A K+-selective, three-state channel from fragmented sarcoplasmic reticulum of frog leg muscle. J Membr Biol 61(1):31–38
Launikonis BS, Zhou J, Royer L, Shannon TR, Brum G, Ríos E (2006) Depletion “skraps” and dynamic buffering inside the cellular calcium store. Proc Natl. Acad Sci USA 103:2982–2987
Manno C, Figueroa LC, Gillespie D, Fitts R, Kang C, Franzini-Armstrong C, Rios E (2017) Calsequestrin depolymerizes when calcium is depleted in the sarcoplasmic reticulum of working muscle. Proc Natl Acad Sci USA 114(4):E638–E647
Melzer W, Ríos E, Schneider MF (1987) A general procedure for determining calcium release in skeletal muscle fibers. Biophys J 51:849–864
Murayama T, Kurebayashi N (2011) Two ryanodine receptor isoforms in nonmammalian vertebrate skeletal muscle: possible roles in excitation-contraction coupling and other processes. Prog Biophys Mol Biol 105(3):134–144
Murayama T, Ogawa Y (2001) Selectively suppressed Ca2+-induced Ca2 + release activity of alpha-ryanodine receptor (alpha-RyR) in frog skeletal muscle sarcoplasmic reticulum: potential distinct modes in Ca2 + release between alpha- and beta-RyR. J Biol Chem 26(4):2953–2960
Naraghi M, Neher E (1997) Linearized buffered Ca2+ diffusion in microdomains and its implications for calculation of [Ca2+] at the mouth of a calcium channel. J Neurosci 17(18):6961–6973
Neher E (1986) Concentration profiles of intracellular calcium in the presence of a diffusible chelator. In: Heinemann U, Klee M, Neher E, Singer W (eds) Calcium electrogenesis and neuronal functioning. Springer Verlag, Berlin, pp 80–96
Olivera JF, Pizarro G (2010) A reappraisal of the Ca2+ dependence of fast inactivation of Ca2+ release in frog skeletal muscle. J Muscle Res Cell Motil 31(2):81–92
Olivera JF, Pizarro G (2016) Excitation contraction uncoupling by high intracellular [Ca2+] in frog skeletal muscle: a voltage clamp study. J Muscle Res Cell Motil 37(4–5):117–130
Pape PC, Carrier N (1998) Effect of sarcoplasmic reticulum (SR) Calciem content on SR Calcium release by small voltage-clamp depolarizations in frog cut skeletal muscle fibers equilibrated with 20 mM EGTA. J Gen Physiol 112:161–179
Pape PC, Jong DS, Chandler WK (1995) Calcium release and its voltage dependence in frog cut muscle fibers equilibrated with 20 mM EGTA. J Gen Physiol 106:259–336
Pape PC, Fénelon K, Carrier N (2002) Extra activation component of calcium release I frog muscle fibers. J Physiol 542(3):867–889
Pape PC, Fénelon K, Lamboley CR, Stachura D (2007) Role of calsequestrin evaluated from changes in free and total calcium concentrations in the sarcoplasmic reticulum of frog cut skeletal muscle fibres. J Physiol 581(Pt 1):319–367
Perni S, Marsden KC, Escobar M, Hollingworth S, Baylor SM, Franzini-Armstrong C (2015) Structural and functional properties of ryanodine receptor type 3 in zebrafish tail muscle. J Gen Physiol 145(3):173–184
Pizarro G, Ríos E (2004) How source content determines intracellular Ca2+ release kinetics. Simultaneous measurement of [Ca2+] transients and [H+] displacement in skeletal muscle. J Gen Physiol 124:239–258
Pouvreau S, Royer L, Yi J, Brum G, Meissner G, Ríos E, Zhou J (2007) Ca2+ sparks operated by membrane depolarization require isoform 3 ryanodine receptor channels in skeletal muscle. Proc Natl Acad Sci USA 104(12):5235–5240
Rios E, Pizarro G (1991) Voltage sensor of excitation-contraction coupling in skeletal muscle. Physiol Rev 71:849–908
Ríos E (2018) Calcium-induced release of calcium in muscle: 50 years of work and the emerging consensus. J Gen Physiol 150(4):521–537
Ríos E, Pizarro G (1988) Voltage sensors and calcium channels of excitation-contraction coupling. NIPS 3:223–227
Ríos E, Karhanek M, Ma J, González A (1993) An allosteric model of the molecular interactions of excitation-contraction coupling in skeletal muscle. J Gen Physiol 102(3):449–481
Robin G, Berthier C, Allard B (2012) Sarcoplasmic reticulum Ca2+ permeation explored from the lumen side in mdx muscle fibers under voltage control. J Gen Physiol 139:209–218
Rudolf R, Magalhães PJ, Pozzan T (2006) Direct in vivo monitoring of sarcoplasmic reticulum Ca2+ and cytosolic cAMP dynamics in mouse skeletal muscle. J Cell Biol 173:187–193
Schneider MF, Simon BJ (1988) Inactivation of calcium release from the sarcoplasmic reticulum in frog skeletal muscle. J Physiol 405:727–745
Shirokova N, Ríos E (1997) Small event Ca2+ release: a probable precursor of Ca2+ sparks in skeletal muscle. J Physiol 502(1):3–11
Shirokova N, García J, Pizarro G, Ríos E (1996) Ca2+ release from the sarcoplasmic reticulum compared in amphibian and mammalian skeletal muscle. J Gen Physiol 107:1–187
Simon BJ, Hill DA (1992) Charge movement and SR calcium release in frog skeletal muscle can be related by a Hodgkin-Huxley model with four gating particles. Biophys J 61(5):1109–1116
Stern MD (1992) Buffering of calcium in the vicinity of a channel pore. Cell Calcium 13:183–192
Stern M, Pizarro G, Ríos E (1997) Local control model of excitation contraction coupling in skeletal muscle. J Gen Physiol 110:415–440
Sutko J, Airey JA (1996) Ryanodine receptor Ca2+ release channels: does diversity in form equal diversity in function? Physiol Rev 76:1027–1071
Sztretye M, Yi J, Figueroa L, Zhou J, Royer L, Ríos E (2011a) D4cpv-calsequestrin: a sensitive ratiometric biosensor targeted to the calcium store of skeletal muscle. J Gen Physiol 138:211–229
Sztretye M, Yi J, Figueroa L, Zhou J, Royer L, Allen PD, Brum G, Ríos E (2011b) Measurement of RyR permeability reveals a role of calsequestrin in termination of SR Ca2+ release in skeletal muscle. J Gen Physiol 138:231–247
Tang S, Wong HC, Wang ZM, Huang Y, Zou J, Zhuo Y, Pennati A, Gadda G, Delbono O, Yang JJ (2011) Design and application of a class of sensors to monitor Ca2+ dynamics in high Ca2+ concentration cellular compartments. Proc Natl Acad Sci USA 108:16265–16270
Tripathy A, Meissner G (1996) Sarcoplasmic reticulum luminal Ca2+ has access to cytosolic activation and inactivation sites of skeletal muscle Ca2+ release channel. Biophys J 70:2600–2615
Ziman AP, Ward CW, Rodney GG, Lederer WJ, Bloch RJ (2010) Quantitative measurement of Ca2+ in the sarcoplasmic reticulum lumen of mammalian skeletal muscle. Biophys J 99:2705–2714
Acknowledgements
This work was funded by PEDECIBA and UdelaR.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Olivera, J.F., Pizarro, G. A study of the mechanisms of excitation–contraction coupling in frog skeletal muscle based on measurements of [Ca2+] transients inside the sarcoplasmic reticulum. J Muscle Res Cell Motil 39, 41–60 (2018). https://doi.org/10.1007/s10974-018-9497-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-018-9497-9