Abstract
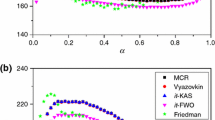
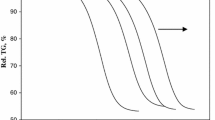
A simple and precise incremental isoconversional integral method based on Li-Tang (LT) method is proposed for kinetic analysis of solid thermal decomposition, in order to evaluate the activation energy as a function of conversion degree. The new method overcomes the limitation of LT method in which the calculated activation energy is influenced by the lower limit of integration. By applying the new method to kinetic analysis of both the simulated nonisothermal case and experimental case of strontium carbonate thermal decomposition, it is shown that the dependence of activation energy on conversion degree evaluated by the new method is consistent with those obtained by Friedman (FR) method and the modified Vyazovkin method. As the new method is free from approximating the temperature integral and not sensitive to the noise of the kinetic data, it is believed to be more convenient in nonisothermal kinetic analysis of solid decompositions.



Similar content being viewed by others
References
Cai JM, Bi LS. Kinetic analysis of wheat straw pyrolysis using isoconversional methods. J Therm Anal Calorim. 2009;98(1):325–30.
Maitra S, et al. Non-isothermal decomposition kinetics of alkaline earth metal carbonates. J Am Ceram Soc. 2007;90(4):1299–303.
Kaljuvee T, et al. Heating rate effect on the thermal behavior of ammonium nitrate and its blends with limestone and dolomite. J Therm Anal Calorim. 2009;97(1):215–21.
Boonchom B. Kinetic and thermodynamic studies of MgHPO4 center dot A 3H(2)O by non-isothermal decomposition data. J Therm Anal Calorim. 2009;98(3):863–71.
Ferriol M, et al. Thermal degradation of poly(methyl methacrylate) (PMMA): modelling of DTG and TG curves. Polym Degrad Stab. 2003;79(2):271–81.
Burnham AK. Computational aspects of kinetic analysis. Part D: the ICTAC kinetics project—multi-thermal-history model-fitting methods and their relation to isoconversional methods. Thermochim Acta. 2000;355(1–2):165–70.
Roduit B. Computational aspects of kinetic analysis. Part E: the ICTAC Kinetics Project—numerical techniques and kinetics of solid state processes. Thermochim Acta. 2000;355(1–2):171–80.
Vyazovkin S. A unified approach to kinetic processing of nonisothermal data. Int J Chem Kinet. 1996;28(2):95–101.
Vyazovkin S, Wight CA. Isothermal and nonisothermal reaction kinetics in solids: In search of ways toward consensus. J Phys Chem A. 1997;101(44):8279–84.
Vyazovkin S. Computational aspects of kinetic analysis. Part C. The ICTAC Kinetics Project—the light at the end of the tunnel? Thermochim Acta. 2000;355(1–2):155–63.
Chen HX, Liu NA. Approximation expressions for the temperature integral. Prog Chem. 2008;20(7–8):1015–20.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38(11):1881–6.
Flynn JH, Wall LA. A quick, direct method for the determination of activation energy from thermogravimetric data. J Polym Sci Part B: Polym Lett. 1966;4(5):323–8.
Coats AW, Redfern JP. Kinetic parameters from thermogravimetric data. Nature. 1964;201(4914):68–9.
Senum GI, Yang RT. Rational approximations of the integral of the Arrhenius function. J Therm Anal Calorim. 1977;11(3):445–7.
Doyle CD. Estimating isothermal life from thermogravimetric data. J Appl Polym Sci. 1962;6(24):639–42.
Chen HX, Liu N, Shu LF. Error of kinetic parameters for one type of integral method for thermogravimetric measurements. Polym Degrad Stab. 2005;90(1):132–5.
Li C-R, Tang TB. Dynamic thermal analysis of solid-state reactions. J Therm Anal Calorim. 1997;49(3):1243–8.
Li CR, Tang TB. Isoconversion method for kinetic analysis of solid-state reactions from dynamic thermoanalytical data. J Mater Sci. 1999;34(14):3467–70.
Li C-R, Tang TB. A new method for analysing non-isothermal thermoanalytical data from solid-state reactions. Thermochim Acta. 1999;325(1):43–6.
Friedman H. Kinetics of thermal degradation of char-forming plastics from thermogravimetry-application to a phenolic resin. J Polym Sci Part C: Polum Lett. 1964;6:183–95.
Budrugeac P, Segal E. On the Li and Tang’s isoconversional method for kinetic analysis of solid-state reactions from thermoanalytical data. J Mater Sci. 2001;36(11):2707–10.
Vyazovkin S. Modification of the integral isoconversional method to account for variation in the activation energy. J Comput Chem. 2001;22(2):178–83.
Ortega A. A simple and precise linear integral method for isoconversional data. Thermochim Acta. 2008;474(1–2):81–6.
Wanjun T, Donghua C. An integral method to determine variation in activation energy with extent of conversion. Thermochim Acta. 2005;433(1–2):72–6.
Budrugeac P, Segal E. On the nonlinear isoconversional procedures to evaluate the activation energy of nonisothermal reactions in solids. Int J Chem Kinet. 2004;36(2):87–93.
Budrugeac P. Differential non-linear isoconversional procedure for evaluating the activation energy of non-isothermal reactions. J Therm Anal Calorim. 2002;68(1):131–9.
Vyazovkin S. Evaluation of activation energy of thermally stimulated solid-state reactions under arbitrary variation of temperature. J Comput Chem. 1997;18(3):393–402.
Acknowledgements
This work was sponsored by National Natural Science Foundation of China under Grant 50806070 and 51076148, and the R&D Special Fund for Public Welfare Industry (forestry, 200704027). Liu Naian was supported by the Fok Ying Tung Education Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, Y., Chen, H. & Liu, N. New incremental isoconversional method for kinetic analysis of solid thermal decomposition. J Therm Anal Calorim 104, 679–683 (2011). https://doi.org/10.1007/s10973-010-1029-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1029-9