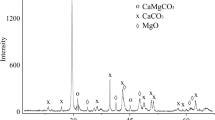
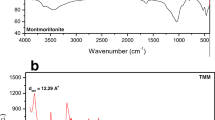
Abstract
In this paper we elaborated the prospective approach for the immobilization of technetium in bentonite clay and Portland cement. It includes thiourea (Tu) addition for Tc effective speciation modification from mobile Tc(VII) to immobile Tc species. Based on the resulting compound structure analysis of Tu with technetium, the reductive mechanism of immobilization was proved. A new technetium complex structure with Tu was described by single crystal XRD analysis, where technetium was obtained in the oxidation state + 3. The complex is stable due to the coordination binding of Tc to the sulfur atoms of the Tu molecules and to the chlorine atom, effective charge transfer along the S–Tc bond, and intramolecular hydrogen bonds. XANES spectra characteristics of technetium (VII, IV, III) reference compounds and samples containing Tc–Tu species in a cement matrix and bentonite clay indicates the presence of technetium in bentonite clay in the form of its tetravalent oxidation state during the Fourier transformation.
Graphical abstract









Similar content being viewed by others
References
Melentev AB, Mashkin AN, German KE (2016). Theor Found Chem Eng. https://doi.org/10.1134/S0040579516040205
Laverov NP, Yudintsev SV, Omel’yanenko BI (2009). Geol Ore Depos. https://doi.org/10.1134/S1075701509040011
Westsik Jr JH, Cantrell KJ, Serne RJ, Qafoku N (2014) Pacific Northwest National Laboratory Richland, Washington, USA: Pacific Northwest National Laboratory Richland. https://doi.org/10.2172/1130666
Isaacs M, Lange S, Deissmann G, Bosbach D, Milodowski AE, Read D (2020). Appl Geochem. https://doi.org/10.1016/j.apgeochem.2020.104580
Eriksen TE, Ndalamba P, Cui D, Bruno J, Caceci M, Spahiu K (1993) SKB Technical Report, TR-93–18. https://www.skb.se/publikation/9249/TR93-18webb.pdf
Warwick P, Aldridge P, Evans N, Vines S (2007). Radiochim Acta. https://doi.org/10.1524/ract.2007.95.12.709
Makarov A, Safonov A, Sitanskaia A, Martynov K, Zakharova E, Kulyukhin S (2022) Clay and carbon materials-based engineered barriers for technetium immobilization. Prog Nucl Energy. https://doi.org/10.1016/j.pnucene.2022.104398
Makarov AV, Safonov AV, Konevnik YV, Teterin YA, Maslakov KI, Teterin AY, Karaseva YY, German KE, Zakharova EV (2021) Activated carbon additives for technetium immobilization in bentonite-based engineered barriers for radioactive waste repositories. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2020.123436
Bruggeman C, et al. (2002) The quantification of the interaction of technetium-99 with dissolved boom clay organic matter
Yuji A, Powell BA, Kaplan DI (2018). J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2017.08.049
Brodda BG (1988). Sci Total Environ. https://doi.org/10.1016/0048-9697(88)90350-6
Kaplan DI, Lilley M, Almond P, Powell BA (2011). SRS. https://doi.org/10.2172/1012465
Um W, Jung HB, Wang G, Westsik JH, Peterson RA (2013) USA: Pacific Northwest National Laboratory Richland. https://doi.org/10.2172/1110479
Lukens WW, Bucher JJ, Shuh DK, Edelstein NM (2005). Environ Sci Technol. https://doi.org/10.1021/cm0622001
Um W, Valenta MM, Chung CW, Yang J, Engelhard MH, Serne RJ, Parker KE, Wang G, Cantrell KJ, Westsik JH (2011) USA: Pacific Northwest National Laboratory Richland. https://doi.org/10.2172/1027193
German KE, Obruchnikova YA, Safonov AV, Tregubova VE, Afanas’ev AV, Kopytin AV, Kryzhovets OS, Poineau F, Abkhalimov EV, Shiryaev AA (2016) Kinetics of the formation of precipitates and the physicochemical properties of technetium-99 and rhenium sulfides according to small-angle x-ray scattering and ultramicrocentrifugation data. Russian J Inorg Chem. https://doi.org/10.1134/S0036023616110061
German KE, Shiryaev AA, Safonov AV, Obruchnikova YA, Ilin VA, Tregubova VE (2015) Technetium sulfide–formation kinetics, structure and particle speciation. Radiochim Acta. https://doi.org/10.1515/ract-2014-2369
Cantrell KJ, Williams BD (2013). J Nucl Mater. https://doi.org/10.1016/j.jnucmat.2013.02.049
Liu Y (2008). Radiochim Acta. https://doi.org/10.1524/ract.2008.1528
Mattigo SV, Lindberg MJ, Westsik JH, Parker KE, Chung CW (2011) USA: Pacific Northwest National Laboratory Richland. https://doi.org/10.2172/1027185
Spence RD, Shi C (2019). CRC. https://doi.org/10.1201/9781420032789
Qafoku NP, Serne RJ, Neeway JJ, Westsik JH, Lawter AR, Valenta Snyder MM, Levitskaia TG (2015) USA: Pacific Northwest National Laboratory Richland. https://doi.org/10.1557/opl.2015.310
Pearce CI et al (2020) Technetium immobilization by materials through sorption and redox-driven processes: a literature review. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2019.06.195
Abrams MJ, Davison A, Faggiani R, Jones AG, Lock CJL (1984). Inorg Chem. https://doi.org/10.1021/ic00189a003
Kopunec R (1979). J Radioanal Nucl Chem. https://doi.org/10.1007/bf02520511
Kamorny DA, Safonov AV, Boldyrev KA, Abramova ES, Tyupina EA, Gorbunova OA (2021). J Nucl Mater. https://doi.org/10.1016/j.jnucmat.2021.153295
Makarov A, Safonov A, Sitanskaia A, Martynov K, Zakharova E, Kulyukhin S (2022). Prog Nucl Energy. https://doi.org/10.1016/j.pnucene.2022.104398
Teterin YA, Makarov AV, Safonov A, Zakharova EV, Maslakov KI, Teterin AY (2021). Inorg Mater. https://doi.org/10.1134/S0020168521090144
Long Time Leach Testing of Solidified Radioactive Waste Forms; Gosstandart of Russia. Moscow, Russia. (2003) 1–8
Krupskaya V, Novikova L, Tyupina E, Belousov P, Dorzhieva O, Zakusin S, Kim K, Roessner F, Badetti E, Brunelli A, Belchinskaya L (2019). Appl Clay Sci. https://doi.org/10.1016/j.clay.2019.02.001
Meleshyn AY, Zakusin SV, Krupskaya VV (2021). Minerals. https://doi.org/10.3390/min11070742
Sheldrick GM (2008) SADABS. Bruker AXS Inc., Madison, Wisconsin, USA
Bruker (2013) SAINT, v. 8.29A, Bruker AXS Inc., Madison, WI
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009). J Appl Cryst. https://doi.org/10.1107/S0021889808042726
Sheldrick GM (2015). Acta Cryst. https://doi.org/10.1107/S2053273314026370
Sheldrick GM (2018) SHELXL-2018. Universität Göttingen, Göttingen (Germany)
Chernyshov AA, Veligzhanin AA, Zubavichus YV (2009). Nucl Inst Meth Phys Res A. https://doi.org/10.1016/j.nima.2008.12.167
Ravel B, Newville M (2005). J Synchrotron Radiat. https://doi.org/10.1107/S0909049505012719
Ankudinov AL, Ravel B, Rehr JJ, Conradson SD (1998). Phys Rev B. https://doi.org/10.1103/PhysRevB.58.7565
Wang Q, German KE, Oganov AR, Dong H, Feya O, Zubavichus YV, Murzin V (2016). RSC Adv. https://doi.org/10.1039/C5RA24656C
Serne RJ, Martin WJ, LeGore VL (1995) PNL-10745 Washington, USA: Pacific Northwest Laboratory Richland. https://doi.org/10.2172/108093
Rochon FD, Melanson R, Kong PC (1996). Polyhedron. https://doi.org/10.1016/0277-5387(95)00534-X
Baldas J, Colmanet SF, Ivanov Z, Williams GA (1994). J Chem Soc Chem Commun. https://doi.org/10.1039/C39940002153
Rochon FD, Melanson R, Kong PC (1992). Inorg Chim Acta. https://doi.org/10.1016/S0020-1693(00)85821-1
Huy NH, Abram U (2007). Inorg Chem. https://doi.org/10.1021/ic070323x
Rochon FD, Melanson R, Kong PC (1990). Acta Crystallogr C. https://doi.org/10.1107/S0108270189008310
Meyer RE, Arnold WD, Case FI (1986). Tech Rep. https://doi.org/10.2172/5954679
Rard JA, Rand MH, Anderegg G, Wanner H (1999) Chemical thermodynamics 3: chemical thermodynamics of technetium. Eds. Sandio MCA, Östhols E. OECD NEA, Data Bank. ELSEVIER. 118. https://www.oecd-nea.org/dbtdb/pubs/vol3-technetium.pdf
Morpurgo L (1968). Inorg Chim Acta. https://doi.org/10.1016/s0020-1693(00)87018-8
Rard JA (2005). J Nucl Radiochem Sci. https://doi.org/10.14494/jnrs2000.6.3_197
Acknowledgements
The authors are grateful to M.S. Grigoriev for invaluable help in analyzing the single crystal data of the Tc-Tu complex. X-ray diffraction experiments were performed at the Center for Shared Use of Physical Methods of Investigation at the Frumkin Institute of Physical Chemistry and Electrochemistry, RAS.
Funding
This work was supported by state assignments from The Ministry of Science and Higher Education of the Russian Federation (#AAAA-A16-11611091001) and performed using the equipment of the Core Facilities Center of IPCE RAS (CKP FMI IPCE RAS). The study was supported by the Ministry of Science and Higher Education of the Russian Federation (program no. 122011300061-3).
Author information
Authors and Affiliations
Contributions
AS: Conceptualization, Investigation, Validation, writing—Original Draft, Supervision, AN: Writing—Original Draft, Investigation, MV: Investigation, Validation, AS: Investigation, Validation, KG: Conceptualization, Methodology, Supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Human or animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Safonov, A., Novikov, A., Volkov, M. et al. Technetium stabilization in Portland cement and bentonite clay barriers by thiourea. J Radioanal Nucl Chem 332, 2195–2204 (2023). https://doi.org/10.1007/s10967-023-08830-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-08830-7