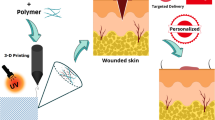
Abstract
The skin is responsible for several necessary physiological activities and plays a prominent role in wound healing. Advanced wound healing requires the use of skin tissue engineering. It was developed primarily to address the difficulties associated with using traditional wound dressings when scars become inflamed or infected. In this way, 3D printing is a unique technology that has attracted an excellent deal of attention within the health profession. This technology improves the ability to combine various medications and proteins with the engineered microstructures of printed products. The advent of 3D printing offers exciting new possibilities for traditional wound dressings to heal broken skin with this new technique. Reconstruction of the skin can be attempted with a specially designed hydrogel with loaded drugs for advanced wound healing. Therefore, this technology can cause a dramatic reduction in the risk of infection and inflammation in wounds. This study comprehensively reviews the latest achievements in polymer 3D printing for wound dressings and skin tissue engineering. Commercially available 3D printing technologies are also explained and these technologies were compared for the wound dressing fabrication.












Similar content being viewed by others
References
Muwaffak Z, Goyanes A, Clark V, Basit AW, Hilton ST, Gaisford S (2017) Patient-specific 3D scanned and 3D printed antimicrobial polycaprolactone wound dressings. Int J Pharm 527(1–2):161–170
Wang W, Lu K-j, Yu C-h, Huang Q-l, Du Y-Z (2019) Nano-drug delivery systems in wound treatment and skin regeneration. J Nanobiotechnology 17(1):1–15
Ahlawat J, Kumar V, Gopinath P (2019) Carica papaya loaded poly (vinyl alcohol)-gelatin nanofibrous scaffold for potential application in wound dressing. Mater Sci Eng C 103:109834
Koehler J, Brandl FP, Goepferich AM (2018) Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur Polymer J 100:1–11
Milne KE, Penn-Barwell JG (2020) Classification and management of acute wounds and open fractures. Surg Infect (Larchmt) 38(3):143–149
Okur ME, Karantas ID, Şenyiğit Z, Okur NÜ, Siafaka PI (2020) Recent trends on wound management: New therapeutic choices based on polymeric carriers. Asian J Pharm Sci
Boateng JS, Matthews KH, Stevens HN, Eccleston GM (2008) Wound healing dressings and drug delivery systems: a review. J Pharm Sci 97(8):2892–2923
Singer AJ, Clark RA (1999) Cutaneous wound healing. N Engl J Med 341(10):738–746
Moeini A, Pedram P, Makvandi P, Malinconico M, d’Ayala GG (2020) Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: a review. Carbohydr Polym 233(115839)
Miguel SP, Moreira AF, Correia IJ (2019) Chitosan based-asymmetric membranes for wound healing: A review. Int J Biol Macromol 127:460–475
Hemmatgir F, Koupaei N, Poorazizi E (2021) Characterization of a novel semi-interpenetrating hydrogel network fabricated by polyethylene glycol diacrylate/polyvinyl alcohol/tragacanth gum as a wound dressing. Burns
Jones A, Vaughan D (2005) Hydrogel dressings in the management of a variety of wound types: A review. J Orthop Nurs 9:S1–S11
Long J, Etxeberria AE, Nand AV, Bunt CR, Ray S, Seyfoddin A (2019) A 3D printed chitosan-pectin hydrogel wound dressing for lidocaine hydrochloride delivery. Mater Sci Eng C 104:109873
Salmanian G, Hassanzadeh-Tabrizi S, Koupaei N (2021) Magnetic chitosan nanocomposites for simultaneous hyperthermia and drug delivery applications: A review. Int J Biol Macromol
Sultan S, Siqueira G, Zimmermann T, Mathew AP (2017) 3D printing of nano-cellulosic biomaterials for medical applications. Curr Opin Biomed Eng 2:29–34
Xu C, Dai G, Hong Y (2019) Recent advances in high-strength and elastic hydrogels for 3D printing in biomedical applications. Acta Biomater 95:50–59
Khoshnood N, Zamanian A (2020) Decellularized extracellular matrix bioinks and their application in skin tissue engineering. Bioprinting:e00095
Groeber F, Holeiter M, Hampel M, Hinderer S, Schenke-Layland K (2011) Skin tissue engineering—in vivo and in vitro applications. Adv Drug Deliv Rev 63(4–5):352–366
Kamel RA, Ong JF, Eriksson E, Junker JP, Caterson EJ (2013) Tissue engineering of skin. J Am Coll Surg 217(3):533–555
Raz-Pasteur A, Fishel R, Hardak E, Mashiach T, Ullmann Y, Egozi D (2016) Do wound cultures give information about the microbiology of blood cultures in severe burn patients? Ann Plast Surg 76(1):34–39
Saghazadeh S, Rinoldi C, Schot M, Kashaf SS, Sharifi F, Jalilian E, Nuutila K, Giatsidis G, Mostafalu P, Derakhshandeh H (2018) Drug delivery systems and materials for wound healing applications. Adv Drug Deliv Rev 127:138–166
Homaeigohar S, Boccaccini AR (2020) Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater 107:25–49
Simões D, Miguel SP, Ribeiro MP, Coutinho P, Mendonça AG, Correia IJ (2018) Recent advances on antimicrobial wound dressing: A review. Eur J Pharm Biopharm 127:130–141
Jin Y-a, Li H, He Y, Fu J-z (2015) Quantitative analysis of surface profile in fused deposition modelling. Addit Manuf 8:142–148
Dhivya S, Padma VV, Santhini E (2015) Wound dressings–a review. BioMedicine 5(4)
Gao M, Sun L, Wang Z, Zhao Y (2013) Controlled synthesis of Ag nanoparticles with different morphologies and their antibacterial properties. Mater Sci Eng, C 33(1):397–404
Sirelkhatim A, Mahmud S, Seeni A, Kaus NHM, Ann LC, Bakhori SKM, Hasan H, Mohamad D (2015) Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-micro Letters 7(3):219–242
Zimbone M, Buccheri M, Cacciato G, Sanz R, Rappazzo G, Boninelli S, Reitano R, Romano L, Privitera V, Grimaldi M (2015) Photocatalytical and antibacterial activity of TiO2 nanoparticles obtained by laser ablation in water. Appl Catal B 165:487–494
Saebnoori E, Koupaei N, Hassanzadeh-Tabrizi S (2021) The solution plasma synthesis, characterisation, and antibacterial activities of dispersed CuO nanoparticles. Mater Tech 1–10
Rahimi M, Hassanzadeh-Tabrizi S, Aminsharei F (2021) Fabrication and antibacterial properties of TFC membrane modified with cellulose/copper oxide nanoparticles for removal of cadmium from water. Sep Sci Technol 1–13
Sriramulu M, Shukla D, Sumathi S (2018) Aegle marmelos leaves extract mediated synthesis of zinc ferrite: antibacterial activity and drug delivery. Mater Res Express 5(11):115404
Udhaya PA, Bessy T, Meena M (2019) Antibacterial activity of nickel and magnesium substituted ferrite nanoparticles synthesized via self-combustion method. Materials Today: Proceedings 8:169–175
Talaei M, Hassanzadeh-Tabrizi S, Saffar-Teluri A (2021) Synthesis of mesoporous CuFe2O4@ SiO2 core-shell nanocomposite for simultaneous drug release and hyperthermia applications. Ceram Int 47(21):30287–30297
Hassanzadeh-Tabrizi S, Norbakhsh H, Pournajaf R, Tayebi M (2021) Synthesis of mesoporous cobalt ferrite/hydroxyapatite core-shell nanocomposite for magnetic hyperthermia and drug release applications. Ceram Int 47(13):18167–18176
Bigham A, Aghajanian AH, Allahdaneh S, Hassanzadeh-Tabrizi S (2019) Multifunctional mesoporous magnetic Mg2SiO4–CuFe2O4 core-shell nanocomposite for simultaneous bone cancer therapy and regeneration. Ceram Int 45(15):19481–19488
Bhaduri B, Engel M, Polubesova T, Wu W, Xing B, Chefetz B (2018) Dual functionality of an Ag-Fe3O4-carbon nanotube composite material: Catalytic reduction and antibacterial activity. J Environ Chem Eng 6(4):4103–4113
Hassanzadeh-Tabrizi S, Behbahanian S, Amighian J (2016) Synthesis and magnetic properties of NiFe2− xSmxO4 nanopowder. J Magn Magn Mater 410:242–247
Hassanzadeh-Tabrizi SA (2019) Mg0. 5Ni0. 5Fe2O4 nanoparticles as heating agents for hyperthermia treatment. J Am Ceram Soc 102(5):2752–2760
Ansari M, Bigham A, Hassanzadeh Tabrizi SA, Abbastabar Ahangar H (2018) Copper-substituted spinel Zn-Mg ferrite nanoparticles as potential heating agents for hyperthermia. J Am Ceram Soc 101(8):3649–3661
Jadhav S, Kim B, Lee H, Im I, Rokade A, Park S, Patil M, Kim G, Yu Y, Lee S (2018) Induction heating and in vitro cytotoxicity studies of MnZnFe2O4 nanoparticles for self-controlled magnetic particle hyperthermia. J Alloy Compd 745:282–291
Beji Z, Hanini A, Smiri L, Gavard J, Kacem K, Villain F, Greneche J-M, Chau F, Ammar S (2010) Magnetic properties of Zn-substituted MnFe2O4 nanoparticles synthesized in polyol as potential heating agents for hyperthermia. Evaluation of their toxicity on Endothelial cells. Chem Mater 22(19):5420–5429
Nasiri M, Tabrizi SAH, Almaki JH, Nasiri R, Idris A, Dabagh S (2016) Synthesis, functionalization, characterization, and in vitro evaluation of robust pH-sensitive CFNs–PA–CaCO 3. RSC Adv 6(87):84217–84230
Elsner JJ, Zilberman M (2009) Antibiotic-eluting bioresorbable composite fibers for wound healing applications: microstructure, drug delivery and mechanical properties. Acta Biomater 5(8):2872–2883
Pachuau L (2015) Recent developments in novel drug delivery systems for wound healing. Expert Opin Drug Deliv 12(12):1895–1909
Tamahkar E, Özkahraman B, Süloğlu AK, İdil N, Perçin I (2020) A novel multilayer hydrogel wound dressing for antibiotic release. J Drug Deliv Sci Technol 58:101536
Llor C, Bjerrum L (2014) Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf 5(6):229–241
Mota RCdAG, da Silva EO, de Lima FF, de Menezes LR, Thiele ACS (2016) 3D printed scaffolds as a new perspective for bone tissue regeneration: literature review. Mater Sci Appl 7(8):430–452
Bracaglia LG, Smith BT, Watson E, Arumugasaamy N, Mikos AG, Fisher JP (2017) 3D printing for the design and fabrication of polymer-based gradient scaffolds. Acta Biomater 56:3–13
Wu G-H, Hsu S-h (2015) polymeric-based 3D printing for tissue engineering. J Med Biol Eng 35(3):285–292
Liu J, Yan C (2018) 3D printing of scaffolds for tissue engineering. by Cvetković D. Intech Open, UK 7:137–154
Wang X, Jiang M, Zhou Z, Gou J, Hui D (2017) 3D printing of polymer matrix composites: A review and prospective. Compos B Eng 110:442–458
Turnbull G, Clarke J, Picard F, Riches P, Jia L, Han F, Li B, Shu W (2018) 3D bioactive composite scaffolds for bone tissue engineering. Bioact Mater 3(3):278–314
Gudapati H, Dey M, Ozbolat I (2016) A comprehensive review on droplet-based bioprinting: past, present and future. Biomaterials 102:20–42
Solomon IJ, Sevvel P, Gunasekaran J (2021) A review on the various processing parameters in FDM. Mater Today Proc 37:509–514
Peng W, Bin Z, Shouling D, Lei L, Huang C (2020) Effects of FDM-3D printing parameters on mechanical properties and microstructure of CF/PEEK and GF/PEEK. Chinese J Aeronaut
Yang C, Tian X, Li D, Cao Y, Zhao F, Shi C (2017) Influence of thermal processing conditions in 3D printing on the crystallinity and mechanical properties of PEEK material. J Mater Process Technol 248:1–7
Wang P, Zou B, Xiao H, Ding S, Huang C (2019) Effects of printing parameters of fused deposition modeling on mechanical properties, surface quality, and microstructure of PEEK. J Mater Process Technol 271:62–74
Rodríguez-Panes A, Claver J, Camacho AM (2018) The influence of manufacturing parameters on the mechanical behaviour of PLA and ABS pieces manufactured by FDM: A comparative analysis. Materials 11(8):1333
Shanmugam V, Das O, Babu K, Marimuthu U, Veerasimman A, Johnson DJ, Neisiany RE, Hedenqvist MS, Ramakrishna S, Berto F (2021) Fatigue behaviour of FDM-3D printed polymers, polymeric composites and architected cellular materials. Int J Fatigue 143:106007
Koupaei N, Karkhaneh A (2016) Porous crosslinked polycaprolactone hydroxyapatite networks for bone tissue engineering. J Tissue Eng Regen Med 13(3):251–260
Koupaei N, Karkhaneh A, Daliri Joupari M (2015) Preparation and characterization of (PCL-crosslinked-PEG)/hydroxyapatite as bone tissue engineering scaffolds. J Biomed Mater Res, Part A 103(12):3919–3926
Zein I, Hutmacher DW, Tan KC, Teoh SH (2002) Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials 23(4):1169–1185
Devi MG, Amutheesan M, Govindhan R, Karthikeyan B (2018) A review of three-dimensional printing for biomedical and tissue engineering applications. Open Biotechnol J 12(1)
Rastogi P, Kandasubramanian B (2019) Breakthrough in the printing tactics for stimuli-responsive materials: 4D printing. Chem Eng J 366:264–304
Elomaa L, Pan C-C, Shanjani Y, Malkovskiy A, Seppälä JV, Yang Y (2015) Three-dimensional fabrication of cell-laden biodegradable poly (ethylene glycol-co-depsipeptide) hydrogels by visible light stereolithography. J Mater Chem B 3(42):8348–8358
Szymczyk-Ziółkowska P, Łabowska MB, Detyna J, Michalak I, Gruber P (2020) A review of fabrication polymer scaffolds for biomedical applications using additive manufacturing techniques. Biocybern Biomed Eng 40(2):624–638
Farzan A, Borandeh S, Ezazi NZ, Lipponen S, Santos HA, Seppälä J (2020) 3D scaffolding of fast photocurable polyurethane for soft tissue engineering by stereolithography: Influence of materials and geometry on growth of fibroblast cells. Eur Polym J 139:109988
Provaggi E, Kalaskar DM (2017) 3D printing families: Laser, powder, nozzle based techniques. 3D Printing in Medicine. Elsevier, pp 21–42
Irvine SA, Agrawal A, Lee BH, Chua HY, Low KY, Lau BC, Machluf M, Venkatraman S (2015) Printing cell-laden gelatin constructs by free-form fabrication and enzymatic protein crosslinking. Biomed Microdevice 17(1):1–8
Pati F, Ha D-H, Jang J, Han HH, Rhie J-W, Cho D-W (2015) Biomimetic 3D tissue printing for soft tissue regeneration. Biomaterials 62:164–175
Varaprasad K, Jayaramudu T, Kanikireddy V, Toro C, Sadiku ER (2020) Alginate-based composite materials for wound dressing application: A mini review. Carbohydr Polym 236:116025
Abasalizadeh F, Moghaddam SV, Alizadeh E, Kashani E, Fazljou SMB, Torbati M, Akbarzadeh A (2020) Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J Biol Eng 14(1):1–22
Chinga-Carrasco G, Ehman NV, Filgueira D, Johansson J, Vallejos ME, Felissia FE, Håkansson J, Area MC (2019) Bagasse—A major agro-industrial residue as potential resource for nanocellulose inks for 3D printing of wound dressing devices. Addit Manuf 28:267–274
Straccia MC, d’Ayala GG, Romano I, Laurienzo P (2015) Novel zinc alginate hydrogels prepared by internal setting method with intrinsic antibacterial activity. Carbohyd Polym 125:103–112
Lee W, Debasitis JC, Lee VK, Lee J-H, Fischer K, Edminster K, Park J-K, Yoo S-S (2009) Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication. Biomaterials 30(8):1587–1595
Binder KW, Allen AJ, Yoo JJ, Atala A (2011) Drop-on-demand inkjet bioprinting: a primer. Gene Ther Regul 6(01):33–49
Chouhan D, Mandal BB (2020) Silk biomaterials in wound healing and skin regeneration therapeutics: From bench to bedside. Acta Biomater 103:24–51
Chen C-S, Zeng F, Xiao X, Wang Z, Li X-L, Tan R-W, Liu W-Q, Zhang Y-S, She Z-D, Li S-J (2018) Three-dimensionally printed silk-sericin-based hydrogel scaffold: a promising visualized dressing material for real-time monitoring of wounds. ACS Appl Mater Interfaces 10(40):33879–33890
Dal Pra I, Chiarini A, Boschi A, Freddi G, Armato U (2006) Novel dermo-epidermal equivalents on silk fibroin-based formic acid-crosslinked three-dimensional nonwoven devices with prospective applications in human tissue engineering/regeneration/repair. Int J Mol Med 18(2):241–247
Goyanes A, Det-Amornrat U, Wang J, Basit AW, Gaisford S (2016) 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J Control Release 234:41–48
Meaume S, Teot L, Lazareth I, Martini J, Bohbot S (2004) The importance of pain reduction through dressing selection in routine wound management: the MAPP study. J Wound Care 13(10):409–413
Streifel BC, Lundin JG, Sanders AM, Gold KA, Wilems TS, Williams SJ, Cosgriff-Hernandez E, Wynne JH (2018) Hemostatic and absorbent PolyHIPE–kaolin composites for 3D printable wound dressing materials. Macromol Biosci 18(5):1700414
Ilhan E, Cesur S, Guler E, Topal F, Albayrak D, Guncu MM, Cam ME, Taskin T, Sasmazel HT, Aksu B (2020) Development of Satureja cuneifolia-loaded sodium alginate/polyethylene glycol scaffolds produced by 3D-printing technology as a diabetic wound dressing material. Int J Biol Macromol 161:1040–1054
Salehi-Abari M, Koupaei N, Hassanzadeh-Tabrizi S (2020) Synthesis and Characterisation of semi-interpenetrating network of Polycaprolactone/polyethylene glycol diacrylate/zeolite-CuO as wound dressing. Mater Technol 35(5):290–299
Wang S, Xiong Y, Chen J, Ghanem A, Wang Y, Yang J, Sun B (2019) Three dimensional printing bilayer membrane scaffold promotes wound healing. Front Bioeng Biotechnol 7:348
Kim BS, Ahn M, Cho W-W, Gao G, Jang J, Cho D-W (2021) Engineering of diseased human skin equivalent using 3D cell printing for representing pathophysiological hallmarks of type 2 diabetes in vitro. Biomaterials 272:120776
Kim BS, Kwon YW, Kong J-S, Park GT, Gao G, Han W, Kim M-B, Lee H, Kim JH, Cho D-W (2018) 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: a step towards advanced skin tissue engineering. Biomaterials 168:38–53
Zhang J, Yun S, Karami A, Jing B, Zannettino A, Du Y, Zhang H (2020) 3D printing of a thermosensitive hydrogel for skin tissue engineering: A proof of concept study. Bioprinting 19:e00089
Abdulhameed O, Al-Ahmari A, Ameen W, Mian SH (2019) Additive manufacturing: Challenges, trends, and applications. Adv Mech Eng 11(2):1687814018822880
Sheoran AJ, Kumar H (2020) Fused Deposition modeling process parameters optimization and effect on mechanical properties and part quality: Review and reflection on present research. Mater Today Proc 21:1659–1672
Dey A, Yodo N (2019) A systematic survey of FDM process parameter optimization and their influence on part characteristics. J Manuf Mater Process 3(3):64
Yuan L, Ding S, Wen C (2019) Additive manufacturing technology for porous metal implant applications and triple minimal surface structures: A review. Bioact Mater 4:56–70
Williams JM, Adewunmi A, Schek RM, Flanagan CL, Krebsbach PH, Feinberg SE, Hollister SJ, Das S (2005) Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 26(23):4817–4827
Gu BK, Choi DJ, Park SJ, Kim MS, Kang CM, Kim C-H (2016) 3-dimensional bioprinting for tissue engineering applications. Biomater Res 20(1):1–8
Mazzoli A (2013) Selective laser sintering in biomedical engineering. Med Biol Eng Compu 51(3):245–256
Landers R, Pfister A, Hübner U, John H, Schmelzeisen R, Mülhaupt R (2002) Fabrication of soft tissue engineering scaffolds by means of rapid prototyping techniques. J Mater Sci 37(15):3107–3116
Rimell JT, Marquis PM (2000) Selective laser sintering of ultra high molecular weight polyethylene for clinical applications. Journal of Biomedical Materials Research: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 53(4):414–420
Schmidt M, Pohle D, Rechtenwald T (2007) Selective laser sintering of PEEK. CIRP Ann 56(1):205–208
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Radmanesh, S., Shabangiz, S., Koupaei, N. et al. 3D printed bio polymeric materials as a new perspective for wound dressing and skin tissue engineering applications: a review. J Polym Res 29, 50 (2022). https://doi.org/10.1007/s10965-022-02899-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-022-02899-6