Abstract
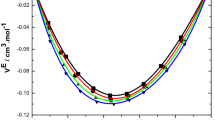
Densities of the ternary system, ethylbenzene + styrene + ethyl acrylate, and its three binaries have been measured in the whole composition range at 298.15 K and atmospheric pressure using an Anton Paar DMA 5000 oscillating U-tube densimeter. The calculated excess molar volumes are positive for the binary system, ethylbenzene + ethyl acrylate, and negative for the systems ethylbenzene + styrene and styrene + ethyl acrylate. The corresponding data were correlated with the Redlich-Kister equation and with a series of Legendre polynomials. Several models were used to correlate the ternary behavior from the excess molar volume data of their constituent binaries and were found to fit the data equally well. The best fit was based on a direct approach, without information on the component binary systems.
Similar content being viewed by others
References
1. Sastry, N.V., Dave, P.N.: Thermodynamics of arylic esters containing binary liquid mixtures. I. Excess volumes and isentropic compressibilities of alky acrylates + n-hexane, + n-heptane, + carbon tetrachloride, + chlorobenzene, and o-dichlorobenzene at 303,15 K. Int. J. Thermophys. 17, 1289–1304 (1996)
2. Sastry, N.V., Dave, P.N.: Dielectric behaviour of acrylic ester—organic solvent mixtures. Proc. Indian Acad. Sci. Chem. Sci. 109, 211–220 (1997)
3. Sastry, N.V., Patel, S.R., Patel, M.C.: Excess volumes, relative permittivity increments, and excess molar polarizations of xCH2CCH3CO2CH3 + (1,x)(C6H6, or C7H8, or o-C8H10, or m-C8H10, or p-C8H10, or C8H10, or c-C6H12). J. Chem. Thermodyn. 31, 797–809 (1999)
4. Peralta, R.D., Infante, R., Cortez, G., Angulo, J.L., Wisniak, J.: Volumetric properties of ethylbenzene with ethyl acrylate, butyl acrylate, methyl methacrylate, and styrene at 298.15 K. Phys. Chem. Liq. 40, 649–660 (2002)
5. Van Ness, H.C., Abbott, M.M.: Classical Thermodynamics of Nonelectrolyte Solutions. McGraw-Hill, New York (1982)
6. Redlich, O., Kister, A.T.: Thermodynamics of nonelectrolytic solutions. Algebraic representation of thermodynamic properties and the classification of solutions. Ind. Eng. Chem. 40, 345–348 (1948)
7. Tomiska, J.: Zur Konversion der anpassungen Thermodynamischer Funktionen mittels einer reihe Legendre'scher Polynome und der Potenzreihe. CALPHAD 5, 93–102 (1981)
8. Tomiska, J.: Mathematical conversions of the thermodynamic excess functions represented by the Redlich-Kister expansion, and by the Chebyshev polynomial series to power series representations and vice-versa. CALPHAD 8, 283–294 (1984)
9. Wisniak, J., Polishuk, A.: Analysis of residues—A useful tool for phase equilibrium data analysis. Fluid Phase Equil. 164, 61–82 (1999)
10. Shacham, M., Wisniak, J., Brauner, N.: Error analysis of linearization methods in regression of data for the van Laar and Margules equations. Ind. Eng. Chem. Res. 32, 2820–2825 (1993)
11. Cibulka, I.: Estimation of excess volume and density of ternary liquid mixtures of nonelectrolytes from binary data. Collect. Czech. Chem Commun. 47, 1414–1419 (1982)
12. Singh, P.P., Nigam, R.K., Sharma, S.O., Aggarwal, S.: Molar excess volumes of ternary mixtures of nonelectrolytes. Fluid Phase Equil. 18, 333–344 (1984)
13. Nagata, I., Tamura, K.: Excess molar enthalpies of methanol or ethanol + (2-butanone + benzene) at 298.15 K. J. Chem. Thermodyn. 22, 279–283 (1990)
14. Tamir, A.: Correlation of vapor-liquid equilibria in systems showing homoazeotropy or heteroazeotropy using expressions for the total pressure and temperature as direct functions of vapor composition. Chem. Eng. Sci. 36, 1467–1473 (1981)
15. TRC, Thermodynamic Tables, extant 2006. Hydrocarbons, Thermodynamics Research Center, The Texas A&M University, College Station, Texas, (a) Table db-3296-0 (April 30, 2001), ethylbenzene, (b) Table d-4490 (April 30, 1989), styrene
16. Gallant, R.W., Yaws, C.L.: Physical Properties of Hydrocarbons, vol. 2, Gulf Publishing Co, Houston, Texas (1993)
17. Sastry, N.V., Valant, M.K.: Volumetric behaviour of acrylic esters (methyl-, ethyl-, and butyl acrylate) + 1-alcohol (heptanol, octanol, decanol, and dodecanol). Phys. Chem. Liq. 38, 61–72 (2000)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wisniak, J., Sandoval, L.E., Peralta, R.D. et al. Density and Volumes of Mixing of the Ternary System Ethylbenzene + Styrene + Ethyl Acrylate and its Binaries at 298.15 K. J Solution Chem 36, 135–152 (2007). https://doi.org/10.1007/s10953-006-9095-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-006-9095-0