Abstract
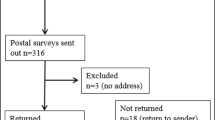
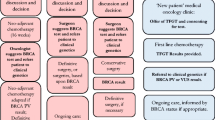
Genetic testing for inherited cancer risk has recently improved through the advent of multi-gene panels and the addition of deletion and duplication analysis of the BRCA genes. The primary aim of this study was to determine which factors influence the intent of individuals with a personal history of breast and/or ovarian cancer and negative or uncertain BRCA1 and BRCA2 testing to return to a hereditary cancer program for additional genetic risk assessment, counseling, and testing. Surveys were sent to 1197 individuals and 257 were returned. Of those participants who were planning to return to clinic, most cited having family members who could benefit from the test result as the primary motivation to return. Many participants who were not planning to return to clinic cited the cost of testing as a barrier to return. Cost of testing and concerns about insurance coverage were the most commonly cited barriers for the group of participants who were undecided about returning to clinic. Results from this study may be used to guide re-contact efforts by clinicians to increase patient uptake to return to clinic for up-to-date genetic risk assessment, counseling, and testing.
Similar content being viewed by others
References
Augusto, E. F., Rosa, M. L., Cavalcanti, S. M., & Oliveira, L. H. (2013). Barriers to cervical cancer screening in women attending the family medical program in Niteroi, Rio de Janeiro. Archives of Gynecology and Obstetrics, 287(1), 53–58. https://doi.org/10.1007/s00404-012-2511-3.
Ayres, L., Kavanaugh, K., & Knafl, K. A. (2003). Within-case and across case approaches to qualitative data analysis. Qualitative Health Research, 13(6), 13.
Castera, L., Krieger, S., Rousselin, A., Legros, A., Baumann, J. J., Bruet, O., et al. (2014). Next-generation sequencing for the diagnosis of hereditary breast and ovarian cancer using genomic capture targeting multiple candidate genes. European Journal of Human Genetics, 22(11), 1305–1313. https://doi.org/10.1038/ejhg.2014.16.
Bakos, A. D., Hutson, S. P., Loud, J. T., Peters, J. A., Giusti, R. M., & Greene, M. H. (2008). BRCA mutation-negative women from hereditary breast and ovarian cancer families: A qualitative study of the BRCA-negative experience. Health Expectations, 11(3), 220–231. https://doi.org/10.1111/j.1369-7625.2008.00494.x.
Buchberg, M. K., Fletcher, F. E., Vidrine, D. J., Levison, J., Peters, M. Y., Hardwicke, R., Yu, X., & Bell, T. K. (2015). A mixed-methods approach to understanding barriers to postpartum retention in care among low-income, HIV-infected women. AIDS Patient Care and STDs, 29, 126–132. https://doi.org/10.1089/apc.2014.0227.
Fecteau, H., Vogel, K. J., Hanson, K., & Morrill-Cornelius, S. (2014). The evolution of cancer risk assessment in the era of next generation sequencing. Journal of Genetic Counseling, 23(4), 633–639. https://doi.org/10.1007/s10897-014-9714-7.
Flores, K. G., Steffen, L. E., McLouth, C. J., Vicuna, B. E., Gammon, A., Kohlmann, W., et al. (2016). Factors associated with interest in gene-panel testing and risk communication preferences in women from BRCA1/2 negative families. Journal of Genetic Counseling, 26, 480–490. https://doi.org/10.1007/s10897-016-0001-7.
Haanpaa, M., Pylkas, K., Moilanen, J.S., & Wingvist, R. (2013). Evaluation of the need for routine clinical testing of PALB2 c.1592delT mutation in BRCA negative Northern Finnish breast cancer families. BMC Medical Genetics, 14(82). http://www.biomedcentral.com/1471-2350/14/82. Accessed 28 Feb 2018.
Hampel, H. (2009). Recontacting patients who have tested negative for BRCA1 and BRCA2 mutations: how, who and why? Journal of Genetic Counseling, 18(6), 527–529. https://doi.org/10.1007/s10897-009-9254-8.
Hiraki, S., Rinella, E. S., Schnabel, F., Oratz, R., & Ostrer, H. (2014). Cancer risk assessment using genetic panel testing: considerations for clinical application. Journal of Genetic Counseling, 23(4), 604–617. https://doi.org/10.1007/s10897-014-9695-6.
Howlader, N., Noone, A.M., Krapcho, M., Miller, D., Bishop, K., Altekruse, S.F., et al. (2016) SEER cancer statistics review, 1975–2013, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April 2016.
Judkins, T., Rosenthal, E., Arnell, C., Burbidge, L. A., Geary, W., Barrus, T., Schoenberger, J., Trost, J., Wenstrup, R. J., & Roa, B. B. (2012). Clinical significance of large rearrangements in BRCA1 and BRCA2. Cancer, 118(21), 5210–5216. https://doi.org/10.1002/cncr.27556.
Krainer, M., Silva-Arrieta, S., FitzGerald, M. G., Shimada, A., Ishioka, C., Kanamaru, R., MacDonald, D. J., Unsal, H., Finkelstein, D. M., Bowcock, A., Isselbacher, K. J., & Haber, D. A. (1997). Differential contributions of BRCA1 and BRCA2 to early-onset breast cancer. The New England Journal of Medicine, 336(20), 1416–1421. https://doi.org/10.1056/nejm199705153362003.
Kurian, A. W., Hare, E. E., Mills, M. A., Kingham, K. E., McPherson, L., Whittemore, A. S., McGuire, V., Ladabaum, U., Kobayashi, Y., Lincoln, S. E., Cargill, M., & Ford, J. M. (2014). Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. Journal of Clinical Oncology, 32(19), 2001–2009. https://doi.org/10.1200/JCO.2013.53.6607.
Landis, J. R., & Koch, G. G. (1977). The measurement of observer agreement for categorical data. Biometrics, 33(1), 159–174.
Mauer, C. B., Pirzadeh-Miller, S. M., Robinson, L. D., & Euhus, D. M. (2014). The integration of next-generation sequencing panels in the clinical cancer genetics practice: an institutional experience. Genetics in Medicine, 16(5), 407–412. https://doi.org/10.1038/gim.2013.160.
Minion, L., Dolinsky, J., Chao, E., & Monk, B. (2014). Hereditary predisposition to ovarian cancer, looking beyond BRCA1/BRCA2. Gynecologic Oncology, 135(2), 383–384. https://doi.org/10.1016/j.ygyno.2014.07.012.
Moran, O., Nikitina, D., Royer, R., Poll, A., Metcalfe, K., Narod, S. A, et al. (2016). Revisiting breast cancer patients who previously tested negative for BRCA mutations using a 12-gene panel. Breast Cancer Research and Treatment, 1–8. doi: 10.1007/s10549-016-4038-y.
Petrucelli, N., Daly, M. B., Pal, T. (2016). BRCA1- and BRCA2-associated hereditary breast and ovarian cancer. 1998 Sep 4 [Updated 2016 Dec 15]. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2017. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1247/.
Pratt, K. J., Collier, D. N., Walton, N. L., Lazorick, S., & Lamson, A. L. (2015). Predictors of follow-up for overweight youth and parents. Families, Systems & Health, 33, 55–60. https://doi.org/10.1037/fsh0000103.
Susswein, L. R., Marshall, M. L., Nusbaum, R., Vogel Postula, K. J., Weissman, S. M., Yackowski, L., Vaccari, E. M., Bissonnette, J., Booker, J. K., Cremona, M. L., Gibellini, F., Murphy, P. D., Pineda-Alvarez, D. E., Pollevick, G. D., Xu, Z., Richard, G., Bale, S., Klein, R. T., Hruska, K. S., & Chung, W. K. (2016). Pathogenic and likely pathogenic variant prevalence among the first 10,000 patients referred for next-generation cancer panel testing. Genetics in Medicine, 18(8), 823–832. https://doi.org/10.1038/gim.2015.166.
Tung, N., Battelli, C., Allen, B., Kaldate, R., Bhatnagar, S., Bowles, K., Timms, K., Garber, J. E., Herold, C., Ellisen, L., Krejdovsky, J., DeLeonardis, K., Sedgwick, K., Soltis, K., Roa, B., Wenstrup, R. J., & Hartman, A. R. (2015). Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer, 121(1), 25–33. https://doi.org/10.1002/cncr.29010.
Vaccari, E., Yackowski, L., Hussong, M., Murphy, P., Cremona, M., Booker, J. et al. (2015). Characterizing the clinical presentation of individuals with pathogrnic variants in a breast/ovarian cancer gene panel: GeneDx.
Wevers, A., Wigboldus, D. H., de Kort, W. L., van Baaren, R., & Veldhuizen, I. J. (2014). Characteristics of donors who do or do not return to give blood and barriers to their return. Blood Transfusion, 12(Suppl 1), s37–s43. https://doi.org/10.2450/2013.0210-12.
Acknowledgements
All research activities were conducted while the first author was enrolled in the Genetic Counseling Program, College of Medicine, University of Cincinnati and Division of Human Genetics, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH. Representatives from GeneDx were not involved in data collection or analysis. The project described was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health, under Award Number 5UL1TR001425-02. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The research team would like to thank Danielle Rolfes and Meghan Tipsword for their contributions to the research, including preparing and sending mailings, scheduling appointments, tracking updated patient contact information, and coding free-response data.
Funding
Funding to conduct this research was provided by GeneDx.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Sara Knapke is an employee of GeneDx, Inc., a wholly owned subsidiary of OPKO Health, Inc.
Sarah E. Chadwell received a research grant from GeneDx, Inc., a wholly owned subsidiary of OPKO Health, Inc. to conduct this research.
Hua He, Jaime Lewis, Rebecca Sisson, and Jennifer Hopper declare that they have no conflicts of interest.
Human Studies and Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Animal Studies
No animal studies were carried out by the authors for this article.
Electronic Supplementary Material
ESM 1
(DOCX 36 kb)
Rights and permissions
About this article
Cite this article
Chadwell, S.E., He, H., Knapke, S. et al. Factors Influencing Clinical Follow-Up for Individuals with a Personal History of Breast and/or Ovarian Cancer and Previous Uninformative BRCA1 and BRCA2 Testing. J Genet Counsel 27, 1210–1219 (2018). https://doi.org/10.1007/s10897-018-0241-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10897-018-0241-9