Abstract
The genesis of cardiogenic oscillations, i.e. the small waves in airway pressure (COSpaw) and flow (COSflow) signals recorded at the airway opening is under debate. We hypothesized that these waves are originated from cyclic changes in pulmonary artery (PA) pressure and flow but not from the physical transmission of heartbeats onto the lungs. The aim of this study was to test this hypothesis. In 10 anesthetized pigs, COS were evaluated during expiratory breath-holds at baseline with intact chest and during open chest conditions at: (1) close contact between heart and lungs; (2) no heart–lungs contact by lifting the heart apex outside the thoracic cavity; (3) PA clamping at the main trunk during 10 s; and (4) during manual massage after cardiac arrest maintaining the heart apex outside the thorax, with and without PA clamping. Baseline COSpaw and COSflow amplitude were 0.70 ± 0.08 cmH2O and 0.51 ± 0.06 L/min, respectively. Both COS amplitude decreased during open chest conditions in step 1 and 2 (p < 0.05). However, COSpaw and COSflow amplitude did not depend on whether the heart was in contact or isolated from the surrounding lung parenchyma. COSpaw and COSflow disappeared when pulmonary blood flow was stopped after clamping PA in all animals. Manual heart massages reproduced COS but they disappeared when PA was clamped during this maneuver. The transmission of PA pulsatilty across the lungs generates COSpaw and COSflow measured at the airway opening. This information has potential applications for respiratory monitoring.
Similar content being viewed by others
1 Introduction
Cardiogenic oscillations (COS) are small waves produced by heartbeats superimposed on gas (COSgas), pressure (COSpaw) and flow (COSflow) signals recorded at the airway opening. Such mechanical waves, represented by COSpaw and COSflow, have important clinical implications. For example, they participate in the process of gas mixing within lungs [1–5], they can be used to assess lung mechanics [6, 7], they can auto-trigger assisted breaths during apnea [8] or they can differentiate patients with central and obstructive sleep apneas [9].
The genesis of COS has been a matter of debate in the past and results from studies are contradictory. On the one hand, several authors concluded that the main cause of COS is the direct physical transfer of heartbeats onto the lungs because both organs are in close contact each other [3, 10–14]. On the other hand, variations in thoracic blood volume during the cardiac cycle or the transmission of pulmonary artery (PA) pulse waves throughout the airways have been related to COS by other researchers [15–18].
We have recently studied the origin of COS in patients undergoing cardiopulmonary bypass, a model that allows to independently manipulate the factors related to COS origin [18]. We observed that COSpaw and COSflow amplitude was not related to the physical contact between heart and lungs but was directly proportional to the increment in pulmonary blood flow. Despite that we have demonstrated that PA pulsatility causes COS in humans, there is still some contradictory information against this theory. In this regards, Fukuchi et al. [4] showed in dogs that COS in nitrogen signal obtained in the right middle lobe persisted when its PA branch was blocked by inflating the balloon of a Swan-Ganz catheter. These data moved us to conduct the present experimental study performing extreme physiological maneuvers ethically impossible to do in humans to provide further evidence about the origin of COS.
Therefore, the main objective of this study was to test the hypothesis that PA pulsatility is the main cause involved in the origin of COSpaw and COSflow. In an experimental model we studied how COSpaw and COSflow were affected by the following maneuvers: (1) interrupting PA blood flow by clamping the main PA artery, (2) totally isolating the heart from lungs lifting the heart apex outside the thorax and (3) performing manual massages after cardiac arrest with the heart apex outside the thorax, with and without PA clamping.
2 Methods
The protocol was approved by the local Ethics Committee for animal experimental research of the Fundación Jiménez Díaz, Madrid, Spain. We studied ten pigs (weight 27 ± 3 kg, length 125 ± 5 cm) anesthetized with a continuous i.v. infusion of propofol 100–150 μg kg min and remifentanyl 1 μg kg min. Saline solution was continuously infused i.v at a rate of 5 mL kg h. The trachea was intubated by a cuffed endotracheal tube and the lungs were mechanically ventilated by a Servoi (Maquet Critical Care, Solna, Sweden) using a constant flow mode with a tidal volume of 7 mL/kg, respiratory rate of 25 bpm, I:E of 1:2, FiO2 of 50 % without positive end-expiratory pressure.
Standard monitoring included ECG, pulse oxymetry, rectal temperature and capnography (NICO; Philips Respironics, Wallingford, CT, USA). Invasive systemic arterial pressure was recorded by a femoral arterial catheter (PV2015L20, Pulsion, Germany). A pulmonary artery catheter was placed through the right jugular vein (Edwards Life-sciences, Irvine, CA, USA). The hemodynamic parameters studied were heart rate (HR), mean arterial pressure (MAP), central venous pressure (CVP), mean pulmonary artery pressure (MPAP), cardiac output (CO) and stroke volume (SV = CO/HR) determined by transpulmonary thermodilution (PiCCO2, Pulsion, Germany). Thermodilution was performed by triplicate before each protocol step.
All described physiologic parameters were recorded continuously and stored in a customized data acquisition system programmed in LabView (National Instruments, Austin TX) during each of the protocol steps.
2.1 Measurement of COSpaw and COSflow
COS were recorded during the protocol performing expiratory breath holds maneuvers by pressing the expiratory hold button of the ventilator for 15 s [18]. We used a dedicated sensor system for measuring airway pressure relative to ambient pressure (HCLA Series, Miniature amplified Low pressure sensors, range ±75 mbar), differential pressure across a Fleisch pneumotachograph. This sensor was placed at the airway opening between the Y piece and the endotracheal tube to assess flow (HCLA Series, Miniature amplified Low pressure sensors, range ±12.5 mbar), and barometric pressure to convert to standard conditions (HCA-BARO Series, Sensor Technics, range 600–1100 mbar)(CSEM, Lanquart, Switzerland). Data from sensors were acquired with a microprocessor and converted into a data stream out of a USB port. The device samples data at 200 Hz for Paw and differential pressure.
2.2 Protocol
After anesthesia induction and animal instrumentation we allowed 30 min for stabilization. Baseline data were recorded at the end of this period in a closed chest condition. Then, the chest was opened by medial sternotomy and the heart was exposed by a sternal retractor after performing a pericardiotomy. In this condition we studied COS in the following sequential steps:
-
1.
Close contact between heart and lungs confirmed by visual inspection.
-
2.
No contact between heart and lungs: the heart was embraced by a soft bandage and then the apex was gently lifted out the thoracic cavity for 15 s.
-
3.
Pulmonary artery clamping: in order to test the role of PA pulse pressure and flow in the origin of COS we clamped the artery for 10 s. This maneuver was performed in two different conditions: a) with close contact between heart and lungs as in point 1 and, b) without contact between heart and lungs by lifting the heart out the thoracic cavity as in point 2.
-
4.
Manual cardiac massages: at the end of the protocol the animal was sacrificed using i.v. potassium chloride. Immediately after cardiac arrest, cardiac massage was performed with the heart lifted out the thoracic cavity (point 2), with and without clamping the PA.
At baseline, steps 1, 2 and 3 the breath hold maneuvers were done by triplicate but at step 4 such maneuver was performed only once. Between each protocol step we introduced at least 5 min of baseline ventilation.
2.3 Data analysis
Raw data of physiologic parameters including ECG, PA pressure, airway flow and pressure were continuously recorded and analyzed off-line. A dedicated software written in Matlab® (Mathworks, Natick, MA, USA) identified COS and ECG signals for their analysis. COS were defined as the small amplitude waves of higher frequency appearing in the pressure and the flow signals between two R waves. Fifteen to twenty COS were analyzed per protocol step depending on the HR. As steps were repeated 3 times in each protocol condition, we analyzed at least 45 COS per step in each animal. The software automatically calculated COSpaw and COSflow amplitude detecting the nadir-to-peak distance of each oscillation corresponding to one cardiac cycle.
We analyzed the hemodynamic data only at baseline (closed chest) and in steps 1 and 2. In steps 3 and 4 we only could describe the presence or absence of COS.
2.4 Statistical analysis
The statistical analysis was performed using the program SPSS (SPSS Inc, Illinois, USA). For comparison of variables between baseline measurements and data from protocol steps 1 and 2 a repeated-measurements analysis of variance was used. If the analysis of variance (F statistic) was significant, the Student–Newman–Keuls post-test was applied. Values are presented as mean ± SD and level of significance was established at p < 0.05.
3 Results
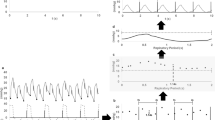
No animal showed COSpaw and COSflow after clamping the PA (Fig. 1). PA pressure signal disappeared during PA clamping maneuver which coincided with an instantaneous disappearance of COS in the pressure and flow signals. This effect was observed when PA clamping was performed with and without contact between heart and lungs (step 3a and 3b, respectively). When PA clamping was released, COSpaw and COSflow reappeared immediately.
Effect of pulmonary artery clamping on cardiogenic oscillations. Two examples of the effect of pulmonary artery clamping on cardiogenic oscillations during close and no contact conditions. Immediately after clamping the PA pulmonary artery pulses, COSpaw and COSflow disappear. After releasing the clamp PA pulsatility and COS reappear concomitantly. Note the corresponding reduction in amplitude of both PA pulsatility and COS after clamp release in the no contact condition
After the administration of i.v. potassium chloride at the end of the experiment, COSpaw and COSflow were reproduced during manual cardiac massage without any contact with the lung parenchyma (step 2, Fig. 2). These artificial COS immediately disappeared when PA was clamped during cardiac massage.
Effect of manual heart massage and PA clamping on cardiogenic oscillations during cardiac arrest. Of notice is the increase in amplitude in COSflow as compared with all other conditions. COS disappear immediately after PA clamping (arrow). PA pulmonary arterial pressure, COS paw cardiogenic oscillations in airway pressure and COS flow cardiogenic oscillations in airway flow. Electrocardiogram (ECG) shows a ventricular fibrillation during this protocol step
The results observed during baseline and protocol steps 1 and 2 with an open chest conditions are presented in Table 1. Compared to baseline values, COSpaw amplitude decreased to 50 % and COSflow amplitude decreased to 37 % when heart and lungs were in close contact. When COS amplitude was compared between baseline and no contact, COSpaw decreased to 59 % and COSflow decreased to 43 % in step 2 (all p < 0.05). We found a small but significant difference in COSpaw amplitude of 18 % between close and no contact.
Hemodynamic data during closed and open chest conditions steps 1 and 2 are presented in Table 2. In general, the hemodynamic state remained stable during baseline measurements and protocol steps 1 and 2. We found a small but significant decrement in SV when no contact was compared with baseline. MPAP was 19 % higher in close contact and 12 % higher in no contact when compared with baseline (p < 0.05). We found no correlation between SV and COS amplitude in any protocol step.
4 Discussion
In this study we provided stronger evidence supporting that COSpaw and COSflow are mainly produced by transmission of pulmonary artery pulsatility. Interrupting pulmonary blood flow after clamping the pulmonary artery eliminated COSpaw and COSflow irrespective of the degree of contact between the beating heart and surrounding lungs. Additionally, manual heart massage during cardiac arrest reproduced artificial COS even when heart was lifted and maintained outside the thorax but disappeared after clamping the pulmonary artery. On the contrary, although there was a small decrease in COSpaw amplitude during no contact, the physical transmission of heartbeats onto the lungs has a minor contribution in COSpaw and no contribution in COSflow origin as both COS persisted after total isolation between heart and lung parenchyma (step 2). These results support our previous findings in human patients and add new evidence regarding the origin of COS answering questions ethically impossible to formulate in humans.
Cyclic changes in intra-thoracic blood volume have been related to COS. This has been explained by a temporal imbalance between the amount of blood ejected outside the thorax by the left ventricle and the inflow received by the right ventricle [11, 16]. However, as the lungs are the main organs within the chest cavity, the pulsatile nature of pulmonary blood flow can be enough to explain the cyclical changes in intra-thoracic volume irrespective of any imbalance between left and right heart volumes. Many authors have demonstrated this pulmonary pulsatility at the pulmonary capillary level [19–21] and similar pulsations have been seen during whole plethysmography [22] or during the recording of nitrous oxide uptake [23]. Furthermore, Montmerle and Linnarsson [16] described how in healthy volunteers COSflow amplitude increased in response to sudden increases in intra-thoracic blood volume after inflation of an anti-gravity suit. They concluded that changes in intra-thoracic volume during the cardiac cycle participate in the genesis of COSflow.
Our previous findings in humans demonstrated a close relationship between COS and the amount of pulmonary blood flow [18]. COSflow and COSpaw doubled their amplitudes when pulmonary blood flow was restored from very low to normal values during cardiopulmonary bypass weaning. Data were obtained with a beating heart under minimal heart-lung contact conditions with the sternal retractor in place. The present results support these findings obtained in humans. We observed COSpaw and COSflow in presence of a normal pulmonary blood flow and pressure but with the beating heart totally isolated from the lungs as in step 2.
The right heart not only creates the pulmonary blood flow but also produces a pulsatile wave that travels along the pulmonary vascular tree [19, 24, 25]. As any mechanical wave, the transmission of this pulsatile wave can occur through different media such as the gas within airways. These transmitted waves can therefore be collected at the airway opening as pressure or flow waveforms with the use of sensors with sufficient sensitivity.
Dahlstrom et al. [15] supported this explanation in an isolated human lung preparation. They reproduced COS in the nitrogram (COSN2) artificially created in the pulmonary vasculature in the absence of any cardiac activity. These data are similar to the ones we observed during the cardiac massage phase of our protocol (Fig. 2) and confirm that the transmission of a mechanical wave, such as the PA pulse pressure wave, is an important factor in the origin of COSpaw and COSflow.
The above data are in opposition to the ones reported by Fukuchi et al. [3] who showed that COSN2 persisted from the right middle lobe when its PA branch was blocked by inflating the balloon of a Swan-Ganz catheter. It is however not easy to fully evaluate Fukuchi’s findings because they performed measurements in only one animal, did not provide any additional hemodynamic data and did not confirm that the vascular occlusion and N2 sampling corresponded to the same lobe. Furthermore, an important difference between Fukuchi’s findings and the data presented here is that we have studied COS as pure mechanical waves (COSpaw and COSflow) while Fukuchi’s analyzed COSN2. COSN2 not only depends on the molecular N2 transport of the mechanical wave but also on N2 concentration stratification among acini due to gravity and lung asymmetry. Thus, possible explanations for their opposing findings could be related to: (1) the pulsatility from right upper and lower lobes made a churning effect on the middle lobe (the one studied), transporting N2 molecules in direction to the sampling catheter. (2) The bronchial vessels, supplied by the aorta, which run alongside the bronchi, caused a churning effect on the right middle lobe [26]. (3) the bronchio-pulmonary anastomoses found at the alveolar level could maintain pulsatility in the right middle lobe distal to the vascular obstruction in the studied dogs [26, 27]. Therefore, COSN2 could be observed despite blood flow interruption in this lobe. In our study pulmonary artery was clamped at its origin thus, pulsatility was stopped in the entire lung parenchyma.
4.1 Limitations
We acknowledge that the results obtained in this experimental setting with an open chest and an open pericardium are not easy to extrapolate to humans with an intact thorax. Despite this non-physiological settings can affect COS amplitude in many complex ways, we believe that the information derived from the extreme maneuvers performed during the presented protocol confirm our hypothesis.
One limitation of our study is that we were unable to acquire thermodilution CO and derived SV data during the 15 s breath-hold maneuver. This means that CO and SV were calculated beat-by-beat using the contour analysis of systemic arterial pressure waveforms (PiCCO2, Pulsion, Germany); a measurement that cannot provide accurate reliable information during these extreme protocol maneuvers. Therefore, the hemodynamic data collected immediately before breath-holds (Table 2) could not represent the real hemodynamic status during this particular moment. We could observe that during the end-expiratory breath-hold continuous pulse contour cardiac output did not change nor did the hemodynamic status per se, as witnessed by stable values of invasive systemic and pulmonary arterial pressures. Furthermore, this limitation may have been of relevance only for the data collected in step 2 as lifting the heart outside the chest could be associated with a more important hemodynamic effect. However, we did not observe any major hemodynamic impairment during this maneuver in the studied animals. Figure 3 illustrates an example of how continuous systemic and pulmonary pressure behaved during step 2 in one of the animals.
Femoral and pulmonary arterial pressures during open-chest and no contact condition (step 2). The figure illustrates the minimal impact on systemic arterial and pulmonary artery pressure amplitude during this stressful protocol condition, when the heart was lifted out of the thoracic cavity and during an expiratory breath hold. FA systemic femoral artery pressure, PA pulmonary arterial pressure and Paw airway pressure. Circle shows the zoom view of cardiogenic oscillations in the airway pressure signal. Despite this aggressive maneuver, electrocardiogram (ECG) confirmed heart activity while PA and FA showed pulmonary and systemic arterial pressures values within a normal range
4.2 Conclusions
The reported data in this study confirm and reinforce that the genesis of COSpaw and COSflow is mainly related to the transmission of pulmonary pulsatility i.e. the cyclic changes in pulmonary blood flow and pressure induced by the right heart activity. Our results, obtained in an open chest condition, suggest a minor contribution of the physical transfer of the heart motion to the surrounding lung parenchyma to the origin of COSpaw and COSflow.
The fact that COS represent the pulmonary artery pulse wave transmission may have interesting implications in lung monitoring and cardiopulmonary interactions. These implication must be analyzed in future studies.
References
Arieli R, Olszowka AJ, Van Liew HD. Postinspiratory mixing in the lungs and cardiogenic oscillations. J Appl Physiol. 1981;51:922–8.
Engel LA, Menkes H, Wood LDH, Utz G, Joubert J, Macklem PT. Gas mixing during breath holding studied by intrapulmonary gas sampling. J Appl Physiol. 1973;35:9–17.
Fukuchi Y, Cosio M, Kelly S, Engel LA. Influence of pericardial fluid on cardiogenic gas mixing in the lungs. J Appl Physiol. 1977;42:5–12.
Fukuchi Y, Roussos C, Macklem PT, Engel LA. Convection, diffusion and cardiogenic mixing of inspired gas in the lungs: an experimental approach. Respir Physiol. 1976;26:77–90.
Slutsky AS. Gas mixing by cardiogenic oscillations: a theoretical quantitative analysis. J Appl Physiol. 1981;51:1287–93.
Bijaoui E, Baconnier PF, Bates JH. Mechanical output impedance of the lung determined from cardiogenic oscillations. J Appl Physiol. 2001;91:859–65.
Lichtwark-Aschoff M, Suki B, Hedlund A, Sjöstrand UH, Markström A, Kawati R, Hedenstierna G, Guttmann J. Decreasing size of cardiogenic oscillations reflects decreasing compliance of the respiratory system during long-term ventilation. J Appl Physiol. 2004;96:879–84.
Imanaka H, Nishimura M, Takeuchi M, Kimball WR, Yahagi N, Kumon K. Autotriggering caused by cardiogenic oscillation during flow-triggered mechanical ventilation. Crit Care Med. 2000;28:402–7.
Ayappa I, Norman RG, Rapoport DM. Cardiogenic oscillations on the airflow signal during continuous positive airway pressure as a marker of central apnea. Chest. 1999;116:660–6.
Arieli R. Cardiogenic oscillations in expired gas: origin and mechanism. Respir Physiol. 1983;52:191–204.
Bosman AR, Lee GD. The effect of cardiac action upon lung gas volume. Clin Sci. 1965;28:311–24.
Butler J. The heart is in good hands. Circulation. 1983;67:1163–77.
Heckman JL, Steward GH, Tremblay G, Lynch PR. Relationship between stroke volume and pneumocardiogram. J Appl Physiol. 1982;52:1672–7.
West JB, Hugh-Jones P. Pulsatile gas flow in bronchi caused by the heart beat. J Appl Physiol. 1961;16:697–702.
Dahlstrom H, Murphy JP, Ross A. Cardiogenic oscillations in composition of expired gases. The pneumocardiogram. J Appl Physiol. 1954;1954(7):335–9.
Montmerle S, Linnarsson D. Effects of gravity and blood volume shifts on cardiogenic oscillations in respired gas. J Appl Physiol. 2005;99:931–6.
Rohdin M, Sundblad P, Linnarsson D. Effects of hypergravity on the distributions of lung ventilation and perfusion in sitting humans assessed with a simple two-step maneuver. J Appl Physiol. 2003;96:1470–7.
Tusman G, Suarez Sipmann F, Peces-Barba G, Climente C, Areta M, Gonzalez Arena P, Bohm SH. Pulmonary blood flow generates cardiogenic oscillations. Respir Physiol. 2009;167:247–54.
Jaryszak EM, Baumgartner WA, Peterson AJ, Presson RG, Glenny RW, Wagner WW. Selected contribution: measuring the response time of pulmonary capillary recruitment to sudden flow changes. J Appl Physiol. 2000;89:1233–8.
Van den Bos GC, Westerhof N, Randall OS. Pulse wave reflection: can it explain the differences between systemic and pulmonary pressure and flow waves? A study in dogs. Circ Res. 1982;51:479–85.
Bloch KE, Jugoon S, Sackner MA. Thoracocardiographic-derived left ventricular systolic time intervals. Chest. 1994;106:1668–74.
DuBois AB, Marshall R. Measurements of pulmonary capillary blood flow and gas exchange throughout the respiratory cycle in man. J Clin Invest. 1957;36:1566–71.
Hollander EH, Wang JJ, Dobson GM, Parker KH, Tyberg JV. Negative wave reflections in pulmonary arteries. Am J Physiol Heart Circ Physiol. 2001;281:H895–902.
Karatzas NB, Noble MIM, Saunders KB, McIlroy MB. Transmission of the blood flow pulse through the pulmonary arterial tree of the dog. Circ Res. 1970;27:1–9.
Brunner HD, Schmidt CF. Blood flow in the bronchial artery of the anesthetized dog. Am J Physiol. 1947;148:648–66.
Charan NB, Albert RK, Lakshminarayan S, Kirk W, Butler J. Factors affecting bronchial blood flow through bronchopulmonary anastomoses in dogs. Am Rev Respir Dis. 1986;134:85–8.
Jindal SK, Lakshminarayan S, Kirk W, Butler J. Acute increase in anastomotic bronchial blood flow after pulmonary arterial obstruction. J Appl Physiol. 1984;57:424–8.
Acknowledgments
We would like to thank Dr. Carlos Castilla head of the experimental laboratory of the Fundación Jiménez Díaz for his highly professional assistance to conduct the experiments and invaluable help in animal preparation and management and Mrs. Pilar Manzano for her skillful assistance. This study was supported by a grant of the “Fondo de Investigaciones Sanitarias” FIS-PI07136.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was performed at the experimental laboratory of the Instituto de investigación Sanitaria, Fundación Jiménez Díaz-UTE (IIS-FJD), Madrid, Spain.
Rights and permissions
About this article
Cite this article
Suarez-Sipmann, F., Santos, A., Peces-Barba, G. et al. Pulmonary artery pulsatility is the main cause of cardiogenic oscillations. J Clin Monit Comput 27, 47–53 (2013). https://doi.org/10.1007/s10877-012-9391-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-012-9391-8