Abstract
Introduction
Non-homologous end joining gene 1 (NHEJ1) defect is a rare form of primary immune deficiency. Very few cases have been described from around the world.
Purpose
We are reporting the first family from the Arabian Gulf with three siblings presenting with combined immunodeficiency (CID), microcephaly, and growth retardation due to a novel NHEJ1 splice site mutation, in addition to a review of the previously published literature on this subject.
Methods
Patients’ clinical, immunological, and laboratory features were examined. Samples were subjected to targeted next-generation sequencing (NGS). The pathogenic change in NHEJ1 was confirmed by Sanger sequencing, then further assessed at the RNA and protein levels.
Results
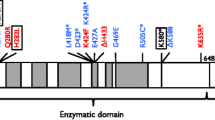
Patients were found to have a homozygous splice site mutation immediately downstream of exon 3 in NHEJ1 (c.390 + 1G > C). This led to two distinct mRNA products, one of which demonstrated skipping of the last 69 basepairs (bp) of exon 3 while the other showed complete skipping of the entire exon. Although both deletions were in-frame, immunoblotting did not reveal any NHEJ1 protein products in patient cells, indicating a null phenotype.
Conclusion
Patients presenting with CID, microcephaly, and growth retardation should be screened for NHEJ1 gene mutations. We discuss our data in the context of one of our patients who is still alive at the age of 30 years, without transplantation, and who is the longest known survivor of this disease.

Similar content being viewed by others
References
Burma S, Chen BP, Chen DJ. Role of non-homologous end joining (NHEJ) in maintaining genomic integrity. DNA Repair (Amst). 2006;5(9–10):1042–8.
Ahnesorg P, Smith P, Jackson SP. XLF interacts with the XRCC4-DNA ligase IV complex to promote DNA nonhomologous end-joining. Cell. 2006;124(2):301–13.
Lu H, et al. Length-dependent binding of human XLF to DNA and stimulation of XRCC4.DNA ligase IV activity. J Biol Chem. 2007;282(15):11155–62.
Tsai CJ, Kim SA, Chu G. Cernunnos/XLF promotes the ligation of mismatched and noncohesive DNA ends. Proc Natl Acad Sci U S A. 2007;104(19):7851–6.
Deshpande RA, Wilson TE. Modes of interaction among yeast Nej1, Lif1 and Dnl4 proteins and comparison to human XLF, XRCC4 and Lig4. DNA Repair (Amst). 2007;6(10):1507–16.
Xing M, et al. Interactome analysis identifies a new paralogue of XRCC4 in non-homologous end joining DNA repair pathway. Nat Commun. 2015;6:6233.
Buck D, et al. Cernunnos, a novel nonhomologous end-joining factor, is mutated in human immunodeficiency with microcephaly. Cell. 2006;124(2):287–99.
Dutrannoy V, et al. Clinical variability and novel mutations in the NHEJ1 gene in patients with a Nijmegen breakage syndrome-like phenotype. Hum Mutat. 2010;31(9):1059–68.
Faraci M, et al. Unrelated hematopoietic stem cell transplantation for Cernunnos-XLF deficiency. Pediatr Transplant. 2009;13(6):785–9.
Al-Mousa H, et al. Unbiased targeted next-generation sequencing molecular approach for primary immunodeficiency diseases. J Allergy Clin Immunol. 2016;137(6):1780–7.
Al-Saud BK, et al. Clinical, immunological, and molecular characterization of hyper-IgM syndrome due to CD40 deficiency in eleven patients. J Clin Immunol. 2013;33(8):1325–35.
Yu Y, et al. DNA-PK and ATM phosphorylation sites in XLF/Cernunnos are not required for repair of DNA double strand breaks. DNA Repair (Amst). 2008;7(10):1680–92.
Gennery AR. Primary immunodeficiency syndromes associated with defective DNA double-strand break repair. Br Med Bull. 2006;77-78:71–85.
IJspeert H, et al. XLF deficiency results in reduced N-nucleotide addition during V(D)J recombination. Blood. 2016;128(5):650–9.
Cagdas D, et al. Two SCID cases with Cernunnos-XLF deficiency successfully treated by hematopoietic stem cell transplantation. Pediatr Transplant. 2012;16(5):E167–71.
Turul T, Tezcan I, Sanal O. Cernunnos deficiency: a case report. J Investig Allergol Clin Immunol. 2011;21(4):313–6.
Carrillo J, et al. Mutations in XLF/NHEJ1/Cernunnos gene results in downregulation of telomerase genes expression and telomere shortening. Hum Mol Genet. 2017;26(10):1900–14.
Patiroglu T, et al. A case of XLF deficiency presented with diffuse large B cell lymphoma in the brain. Clin Immunol. 2015;161(2):394–5.
Cantagrel V, et al. Truncation of NHEJ1 in a patient with polymicrogyria. Hum Mutat. 2007;28(4):356–64.
Avagyan S, et al. Hematopoietic stem cell dysfunction underlies the progressive lymphocytopenia in XLF/Cernunnos deficiency. Blood. 2014;124(10):1622–5.
Meyer-Bahlburg A, Dressler F, Baumann U. Chronic arthritis in a boy with Cernunnos immunodeficiency. Clin Immunol. 2014;154(1):47–8.
Cipe FE, et al. Cernunnos/XLF deficiency: a syndromic primary immunodeficiency. Case Rep Pediatr. 2014;2014:614238.
Slack J et al. Outcome of hematopoietic cell transplantation for DNA double-strand break repair disorders. J Allergy Clin Immunol. 2017.
Malivert L, et al. Delineation of the Xrcc4-interacting region in the globular head domain of cernunnos/XLF. J Biol Chem. 2010;285(34):26475–83.
Acknowledgements
We thank all family members for their enthusiastic participation. We acknowledge the Saudi Human Genome Project for infrastructure and informatics support relating to the targeted NGS. We are also grateful to all the immunology staff, and to the sequencing core facility, at KFSH&RC for their invaluable assistance. This work was supported by the National Science, Technology and Innovation Plan program of Saudi Arabia (KACST 14-MED316-20).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sheikh, F., Hawwari, A., Alhissi, S. et al. Loss of NHEJ1 Protein Due to a Novel Splice Site Mutation in a Family Presenting with Combined Immunodeficiency, Microcephaly, and Growth Retardation and Literature Review. J Clin Immunol 37, 575–581 (2017). https://doi.org/10.1007/s10875-017-0423-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-017-0423-5