Abstract
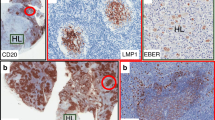
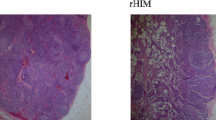
Given the severely reduced numbers of circulating class-switched memory B cells and plasmablasts in patients with common variable immunodeficiency (CVID) the germinal center (GC) reaction as the source of both populations is expected to be disturbed in many CVID patients. Therefore immunohistochemical studies were performed on lymph node (LN) biopsies from ten CVID patients with benign lymphoproliferation. According to the Sander classification the majority of patients presented with reactive lymphoid hyperplasia (7/10), 6/10 showed granulomatous inflammation. All cases showed some normal GCs but in 9/10 these concurred to a varying degree with hyperplastic, ill-defined GCs in the same LN. The percentage of ill-defined GCs correlated significantly with the percentage of circulating CD21low B cells suggesting a common origin of both immune reactions. In 9/10 CVID LNs significantly higher numbers of infiltrating CD8+ T cells were found in GCs of CVID patients compared to controls, but no HHV-8 and only in 2/10 LNs EBV infection was detected. Class switched plasma cells (PCs) were severely reduced in 8/10 LNs and if present, rarely found in the medulla of the LN. Based on the presence of large GCs in all examined patients, the reduction of circulating memory B cells and PCs points towards a failure of GC output rather than GC formation in CVID patients with lymphadenopathy.




Similar content being viewed by others
References
Warnatz K, Denz A, Drager R, Braun M, Groth C, Wolff-Vorbeck G, et al. Severe deficiency of switched memory B cells (CD27(+)IgM(−)IgD(−)) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood. 2002;99(5):1544–51.
DiSanto JP, Bonnefoy JY, Gauchat JF, Fischer A, de Saint BG. CD40 ligand mutations in x-linked immunodeficiency with hyper-IgM. Nature. 1993;361(6412):541–3.
Ferrari S, Giliani S, Insalaco A, Al-Ghonaium A, Soresina AR, Loubser M, et al. Mutations of CD40 gene cause an autosomal recessive form of immunodeficiency with hyper IgM. Proc Natl Acad Sci U S A. 2001;98(22):12614–9.
Warnatz K, Bossaller L, Salzer U, Skrabl-Baumgartner A, Schwinger W, van der Burg M, et al. Human ICOS deficiency abrogates the germinal center reaction and provides a monogenic model for common variable immunodeficiency. Blood. 2006;107(8):3045–52.
Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455(7214):764–9.
Revy P, Muto T, Levy Y, Geissmann F, Plebani A, Sanal O, et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2). Cell. 2000;102(5):565–75.
Wehr C, Kivioja T, Schmitt C, Ferry B, Witte T, Eren E, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111(1):77–85.
Sander CA, Medeiros LJ, Weiss LM, Yano T, Sneller MC, Jaffe ES. Lymphoproliferative lesions in patients with common variable immunodeficiency syndrome. Am J Surg Pathol. 1992;16(12):1170–82.
Groth C, Drager R, Warnatz K, Wolff-Vorbeck G, Schmidt S, Eibel H, et al. Impaired up-regulation of CD70 and CD86 in naive (CD27-) B cells from patients with common variable immunodeficiency (CVID). Clin Exp Immunol. 2002;129(1):133–9.
Fischer MB, Hauber I, Eggenbauer H, Thon V, Vogel E, Schaffer E, et al. A defect in the early phase of T-cell receptor-mediated T-cell activation in patients with common variable immunodeficiency. Blood. 1994;84(12):4234–41.
Wheat WH, Cool CD, Morimoto Y, Rai PR, Kirkpatrick CH, Lindenbaum BA, et al. Possible role of human herpesvirus 8 in the lymphoproliferative disorders in common variable immunodeficiency. J Exp Med. 2005;202(4):479–84.
Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies). Clin Immunol. 1999;93(3):190–7.
Kojima M, Nakamura S, Itoh H, Motoori T, Sugihara S, Shinkai H, et al. Angioimmunoblastic T-cell lymphoma with hyperplastic germinal centers: a clinicopathological and immunohistochemical study of 10 cases. APMIS. 2001;109(10):699–706.
Toccanier MF, Kapanci Y. Lymphadenopathy in drug addicts. A study of the distribution of T lymphocyte subsets in the lymph nodes. Virchows Archiv A, Pathological Anat Histopathol. 1985;406(2):149–63.
O’Malley DPGT, Orazi A, Abbondanzo SL. General Reactive Conditions in Lymph Node and Spleen. In: O’Malley DPGT, Orazi A, Abbondanzo SL, editors. Atlas of Nontumor Pathology 7 Benign & Reactive Conditions Lymph Node & Spleen. 1st ed. Washington, DC: The American Registry of Pathology; 2009. p. 129–33.
Chan PK, Ng HK, Cheung JL, Cheng AF. Survey for the presence and distribution of human herpesvirus 8 in healthy brain. J Clin Microbiol. 2000;38(7):2772–3.
Warnatz K, Schlesier M. Flowcytometric phenotyping of common variable immunodeficiency. Cytometry B Clin Cytom. 2008;74(5):261–71.
Chevalier N, Jarrossay D, Ho E, Avery DT, Ma CS, Yu D, et al. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J Immunol. 2011;186(10):5556–68.
Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34(1):108–21.
Ochtrop ML, Goldacker S, May AM, Rizzi M, Draeger R, Hauschke D, et al. T and B lymphocyte abnormalities in bone marrow biopsies of common variable immunodeficiency. Blood. 2011;118(2):309–18.
Taubenheim N, von Hornung M, Durandy A, Warnatz K, Corcoran L, Peter HH, et al. Defined blocks in terminal plasma cell differentiation of common variable immunodeficiency patients. J Immunol. 2005;175(8):5498–503.
Herbst EW, Armbruster M, Rump JA, Buscher HP, Peter HH. Intestinal B cell defects in common variable immunodeficiency. Clin Exp Immunol. 1994;95(2):215–21.
Scott LJ, Bryant A, Webster AD, Farrant J. Failure in IgA secretion by surface IgA-positive B cells in common variable immunodeficiency (CVID). Clin Exp Immunol. 1994;95(1):10–3.
Fossum S, Ford WL. The organization of cell populations within lymph nodes: their origin, life history and functional relationships. Histopathology. 1985;9(5):469–99.
van der Valk P MC. The Lymph Nodes. In: Mills SE, editors. Histology for Pathologists. 3 ed. Philadelphia: Lippincott Williams and Wilkins; 2007. pp. 763–81.
Humpert ML, Pinto D, Jarrossay D, Thelen M. CXCR7 influences the migration of B cells during maturation. European journal of immunology. 2013.
Kojima M, Kashimura M, Itoh H, Noro M, Matsuda H, Tsukamoto N. Infectious mononucleosis lymphoadenitis showing histologic findings indistinguishable from toxoplasma lymphadenitis. A report of three cases. Pathol Res Pract. 2010;206(6):361–4.
Gujral S, Gandhi JS, Valsangkar S, Shet TM, Epari S, Subramanian PG. Study of the morphological patterns and association of Epstein-Barr virus and human herpes virus 8 in acquired immunodeficiency deficiency syndrome-related reactive lymphadenopathy. Indian J Pathol Microbiol. 2010;53(4):723–8.
Turner RR, Levine AM, Gill PS, Parker JW, Meyer PR. Progressive histopathologic abnormalities in the persistent generalized lymphadenopathy syndrome. Am J Surg Pathol. 1987;11(8):625–32.
Kojima M, Kitamoto Y, Shimizu K, Matsuda H, Masawa N. Tonsillar lesions of infectious mononucleosis resembling MALT type lymphoma. A report of two cases. Pathol Oncol Res: POR. 2008;14(4):489–92.
Mrusek S, Marx A, Kummerle-Deschner J, Tzaribachev N, Enders A, Riede UN, et al. Development of granulomatous common variable immunodeficiency subsequent to infection with Toxoplasma gondii. Clin Exp Immunol. 2004;137(3):578–83.
Siim JC, Nissen NI. Toxoplasmosis acquisita lymphonodosa in a 62-year-old woman; isolation of Toxoplasma gondli from lymph node and muscle biopsies. Acta Pathol Microbiol Scand. 1958;43(3):298–304.
Sheibani K, Fritz RM, Winberg CD, Burke JS, Rappaport H. “Monocytoid” cells in reactive follicular hyperplasia with and without multifocal histiocytic reactions: an immunohistochemical study of 21 cases including suspected cases of toxoplasmic lymphadenitis. Am J Clin Pathol. 1984;81(4):453–8.
Dargent JL, Haller A, Durdurez JP, Gennotte AF. Atypical hyperplasia of the marginal zone of B follicles in a polymorphic Epstein-Barr virus-associated lymphoproliferative disorder occurring in an adolescent with human immunodeficiency virus infection. Pediatr Dev Pathol: the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. 2009;12(1):59–62.
Ree HJ, Kadin ME, Kikuchi M, Ko YH, Go JH, Suzumiya J, et al. Angioimmunoblastic lymphoma (AILD-type T-cell lymphoma) with hyperplastic germinal centers. Am J Surg Pathol. 1998;22(6):643–55.
Ree HJ, Kadin ME, Kikuchi M, Ko YH, Suzumiya J, Go JH. Bcl-6 expression in reactive follicular hyperplasia, follicular lymphoma, and angioimmunoblastic T-cell lymphoma with hyperplastic germinal centers: heterogeneity of intrafollicular T-cells and their altered distribution in the pathogenesis of angioimmunoblastic T-cell lymphoma. Hum Pathol. 1999;30(4):403–11.
Dezube BJ, Aboulafia DM, Pantanowitz L. Plasma cell disorders in HIV-infected patients: from benign gammopathy to multiple myeloma. AIDS Read. 2004;14(7):372–4. 7–9.
O’Murchadha MT, Wolf BC, Neiman RS. The histologic features of hyperplastic lymphadenopathy in AIDS-related complex are nonspecific. Am J Surg Pathol. 1987;11(2):94–9.
Zhang Y, Meyer-Hermann M, George LA, Figge MT, Khan M, Goodall M, et al. Germinal center B cells govern their own fate via antibody feedback. J Exp Med. 2013;210(3):457–64.
Mouillot G, Carmagnat M, Gerard L, Garnier JL, Fieschi C, Vince N, et al. B-cell and T-cell phenotypes in CVID patients correlate with the clinical phenotype of the disease. J Clin Immunol. 2010;30(5):746–55.
Rakhmanov M, Keller B, Gutenberger S, Foerster C, Hoenig M, Driessen G, et al. Circulating CD21low B cells in common variable immunodeficiency resemble tissue homing, innate-like B cells. Proc Natl Acad Sci U S A. 2009;106(32):13451–6.
Boursiquot JN, Gerard L, Malphettes M, Fieschi C, Galicier L, Boutboul D, et al. Granulomatous disease in CVID: retrospective analysis of clinical characteristics and treatment efficacy in a cohort of 59 patients. J Clin Immunol. 2013;33(1):84–95.
Al Kindi M, Mundy J, Sullivan T, Smith W, Kette F, Smith A, et al. Utility of peripheral blood B cell subsets analysis in common variable immunodeficiency. Clin Exp Immunol. 2012;167(2):275–81.
Asano S. Granulomatous lymphadenitis. J Clin Exp Hematopathology : JCEH. 2012;52(1):1–16.
Kuntz M, Goldacker S, Blum HE, Pircher H, Stampf S, Peter HH, et al. Analysis of bulk and virus-specific CD8+ T cells reveals advanced differentiation of CD8+ T cells in patients with common variable immunodeficiency. Clin Immunol. 2011;141(2):177–86.
Kim HJ, Verbinnen B, Tang X, Lu L, Cantor H. Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature. 2010;467(7313):328–32.
Quigley MF, Gonzalez VD, Granath A, Andersson J, Sandberg JK. CXCR5+ CCR7–CD8 T cells are early effector memory cells that infiltrate tonsil B cell follicles. Eur J Immunol. 2007;37(12):3352–62.
Racz P, Tenner-Racz K, van Vloten F, Schmidt H, Dietrich M, Gluckman JC, et al. Lymphatic tissue changes in AIDS and other retrovirus infections: tools and insights. Lymphology. 1990;23(2):85–91.
Keller AR, Hochholzer L, Castleman B. Hyaline-vascular and plasma-cell types of giant lymph node hyperplasia of the mediastinum and other locations. Cancer. 1972;29(3):670–83.
Schulte KM, Talat N. Castleman’s disease–a two compartment model of HHV8 infection. Nat Rev Clin Oncol. 2010;7(9):533–43.
Acknowledgments
We would like to thank Mrs. Weinhold for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Susanne Unger and Maximilian Seidl contributed equally to this work.
This study was supported by the German Federal Ministry of Education and Research (BMBF 01 EO 1303). The authors are responsible for the contents of this publication (S.U., M.S., K.W.)
Rights and permissions
About this article
Cite this article
Unger, S., Seidl, M., Schmitt-Graeff, A. et al. Ill-Defined Germinal Centers and Severely Reduced Plasma Cells are Histological Hallmarks of Lymphadenopathy in Patients with Common Variable Immunodeficiency. J Clin Immunol 34, 615–626 (2014). https://doi.org/10.1007/s10875-014-0052-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-014-0052-1