Abstract
Background
Busulfan treatment as a chemotherapeutic agent has been considered an alternative approach in xenograft model because it offers a simple, convenient, effective, and less toxic conditioning regimen.
Objective and methods
To investigate busulfan effects on the reconstitution of human immune cells and the generation of immune response to foreign antigens, we generated humanized NOD/SCID/IL-2Rγnull (NSG) mice conditioned either busulfan or total body irradiation (TBI) with hCD34+ CB cells.
Results
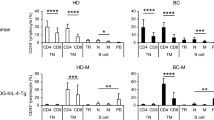
Busulfan resulted in a high survival rate and effective reconstitution of human immune cells including B, T, macrophage, and dendritic cells in humanized NSG mice, compared to that of TBI. Moreover, the humanized NSG mice conditioned busulfan showed effective B cell development and thereby the high production of human antibody against immunized antigen.
Conclusion
Humanized mice conditioned by busulfan provide a powerful and versatile tool for studying the entire process of human B-lymphocyte development and for producing specific human antibodies








Similar content being viewed by others
References
Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–30.
Manz MG. Human-hemato-lymphoid-system mice: opportunities and challenges. Immunity. 2007;26:537–41.
Macchiarini F, Manz MG, Palucka AK, Shultz LD. Humanized mice: are we there yet? J Exp Med. 2005;202:1307–11.
Payne KJ, Crooks GM. Immune-cell lineage commitment: translation from mice to humans. Immunity. 2007;26:674–7.
Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, Othieno FA, et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006;12:1316–22.
Mosier DE, Gulizia RJ, Baird SM, Wilson DB. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335:256–9.
Kaneshima H, Namikawa R, McCune JM. Human hematolymphoid cells in SCID mice. Curr Opin Immunol. 1994;6:327–33.
Pflumio F, Izac B, Katz A, Shultz LD, Vainchenker W, Coulombel L. Phenotype and function of human hematopoietic cells engrafting immune-deficient CB17-severe combined immunodeficiency mice and nonobese diabetic-severe combined immunodeficiency mice after transplantation of human cord blood mononuclear cells. Blood. 1996;88:3731–40.
Shultz LD, Lang PA, Christianson SW, Gott B, Lyons B, et al. NOD/LtSz-Rag1null mice: an immunodeficient and radioresistant model for engraftment of human hematolymphoid cells, HIV infection, and adoptive transfer of NOD mouse diabetogenic T cells. J Immunol. 2000;164:2496–507.
Goldman JP, Blundell MP, Lopes L, Kinnon C, Di Santo JP, Thrasher AJ. Enhanced human cell engraftment in mice deficient in RAG2 and the common cytokine receptor gamma chain. Br J Haematol. 1998;103:335–42.
Kollet O, Peled A, Byk T, Ben-Hur H, Greiner D, Shultz L, et al. β2 Microglobulin-deficient (B2mnull) NOD/SCID mice are excellent recipients for studying human stem cell function. Blood. 2000;95:3102–5.
Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, et al. NOD/SCID/γ nullc mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–82.
Hiramatsu H, Nishikomori R, Heike T, Ito M, Kobayashi K, Katamura K, et al. Complete reconstitution of human lymphocytes from cord blood CD34+ cells using the NOD/SCID/γ nullc mice model. Blood. 2003;102:873–80.
Haddow A, Timmis GM. Myleran in chronic myeloid leukaemia; chemical constitution and biological action. Lancet. 1953;31:207–8.
Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–89.
Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor γ chainnull mice. Blood. 2005;106:1565–73.
Ciurea SO, Andersson BS. Busulfan in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:523–36.
Mauch P, Down JD, Warhol M, Hellman S. Recipient preparation for bone marrow transplantation. I. Efficacy of total-body irradiation and busulfan. Transplantation. 1988;46:205–10.
Down JD, Ploemacher RE. Transient and permanent engraftment potential of murine hematopoietic stem cell subsets: differential effects of host conditioning with gamma radiation and cytotoxic drugs. Exp Hematol. 1993;21:913–21.
Hsieh MM, Langemeijer S, Wynter A, Phang OA, Kang EM, Tisdale JF. Low-dose parenteral busulfan provides an extended window for the infusion of hematopoietic stem cells in murine hosts. Exp Hematol. 2007;35:1415–20.
Hayakawa J, Hsieh MM, Uchida N, Phang O, Tisdale JF. Busulfan produces efficient human cell engraftment in NOD/LtSz-Scid IL2Rγ null mice. Stem Cells. 2009;27:175–82.
Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol. 2005;5:230–42.
Allman D, Pillai S. Peripheral B cell subsets. Curr Opin Immunol. 2008;20:149–57.
Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunol Rev. 2004;197:179–91.
Tonomura N, Habiro K, Shimizu A, Sykes M, Yang YG. Antigen-specific human T-cell responses and T cell-dependent production of human antibodies in a humanized mouse model. Blood. 2008;111:4293–6.
McCune JS, Holmberg LA. Busulfan in hematopoietic stem cell transplant setting. Expert Opin Drug Metab Toxicol. 2009;8:957–69.
Chen J. Animal models for acquired bone marrow failure syndromes. Clin Med Res. 2005;2:102–8.
Sauer M, Zeidler C, Meissner B, Rehe K, Hanke A, Welte K, et al. Substitution of cyclophosphamide and busulfan by fludarabine, treosulfan and melphalan in a preparative regimen for children and adolescents with Shwachman–Diamond syndrome. Bone Marrow Transplant. 2007;39:143–7.
Boyd RL, Caro J, Halka KG, Erslev AJ. Granulopoiesis in long-term culture by marrow from mice with busulfan-induced chronic latent aplasia. Int J Cell Cloning. 1986;4:357–67.
Acknowledgment
This work was supported by Mid-career Researcher Program through NRF grant funded by the MEST (2009-0084573; to K.-Y.L.), the Korea Health 21 R&D Project, Ministry of Health and Welfare (A080568; to S.J.K.), and by Medical Research Foundation of Samsung Biomedical Research Institute (SBRI) grant (C-A6-407-1; to S.J.K.).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Bongkum Choi, Eunyoung Chun, and Miyoung Kim are equally contributed to this work
Rights and permissions
About this article
Cite this article
Choi, B., Chun, E., Kim, M. et al. Human B Cell Development and Antibody Production in Humanized NOD/SCID/IL-2Rγnull (NSG) Mice Conditioned by Busulfan. J Clin Immunol 31, 253–264 (2011). https://doi.org/10.1007/s10875-010-9478-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-010-9478-2