Abstract
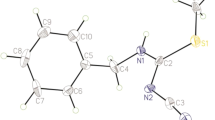
The oximino(2,6-dichlorophenyl)acetonitrile, H(2,6-diCl-PhCO) has been synthesized in a reasonably high yield of 60%, and characterized using a variety of physical, electrochemical, spectroscopic methods and X-ray analysis. This compound belongs to the family of cyanoximes; a new subclass of oximes with the general formula NC–C(=N–OH)–R (where R is an electron-withdrawing group) which recently emerged as new biologically active compounds. This cyanoxime represents a disubstituted arylcyanoxime that was found to be a powerful inhibitor of the Carbonyl Reductase enzyme involved in the developing of resistance to anticancer treatment, and the making of cardiotoxic derivatives of anthracyclines that are currently used in medicine. The oximino(2,6-dichlorophenyl)acetonitrile, H(2,6-diCl-PhCO) is a weak acid with pKa = 6.17 and does not dissociate in organic polar protic and aprotic solvents. The cyanoxime was obtained as a microcrystalline mixture of two diastereomers (anti- and syn-) and deprotonates in solutions with the formation of yellow anions which exhibit solvatochromic behavior. However, one specific diastereomer—syn—was isolated in crystalline form from a solvent system as colorless blocks overlayed with pentane ether solution in a monoclinic system in a P2/c (#13) space group with unit cell parameters: a = 8.1720(2), b = 8.8013(3), c = 13.0146(4) and β = 102.546(3); Z = 4. A single crystal was studied using filtered CuKa radiation, providing Rint value of 0.0348 from a full-sphere of reflections. A crystal structure was solved using direct methods, and well refined to R1 = 0.0459, wR2 = 0.1268 and GOF = 1.107. The studied specimen of oximino(2,6-dichlorophenyl)acetonitrile, H(2,6-diCl-PhCO), represents a highly non-planar, rare syn-diastereomer in which the oxime fragment is positioned towards the chlorinated phenyl group. In the crystal, the compound forms a columnar structure extended along the c-direction by using slipped π–π stacking interactions. Columns are interconnected via H-bonding between the oxime OH-group and N atom of the nitrile group with the following parameters: N–H = 1.841 Å, and 169.20° N···H–O angle. No thermal interconversion of syn- into anti- diastereomer was observed upon heating of crystals of one isomer under flow of Ar.
Graphic Abstract






Similar content being viewed by others
References
Minotti G et al (2004) Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardioitoxicity. Pharmacol Rev 56:185–229
Olson et al (2003) Protection from doxorubicin-induced cardiac toxicity in mice with a null allele of carbonyl reductase 1. Cancer Res 63:6602–6606
Forrest et al (2000) Human carbonyl reductase overexpression in the heart advances the development of doxorubicin-induced cardiotoxicity in transgenic mice. Cancer Res 60:5158–5164
Tanaka et al (2005) An unbiased cell morphology-based screen for new, biologically active small molecules. PLoS Biol 3(5):0764–0776
Tirapazamine: radiation sensitizer and chemotherapy anticancer drug in combination with cisplatin in Phase I and Phase II clinical trials by National Cancer Institute (2005)
Carmustine® and Lomustine®: anticancer drugs by Bristol-Myers Squibb; Physician Desk Reference, 51 ed, pp.696–699 (1997).
Mongin F, Trecourt F, Gervais B, Mongin O, Queguinner G (2002) J Org Chem 67:3272–3279
Kists HA, Szymanski EF, Dorman DEJ (1975) Antibiotics 28(4):286–291
Nakamura H, Iitaka Y, Sakakibara H, Umezawa HJ (1974) Antibiotics 27(11):894–896
New carbamoyl oximes, their preparation and compositions containing them. Patent of Switzerland #1244497, 1968.
Geigy AGC (1985) Srodek ochriny roslin przed dzialaniem agresywnych chemikalii rolniczych. Patent of Poland #127786
Geigy AGC (1982) Mittel zum Schutz von Kulturpflanzen von agressiven Herbiziden. Patent of Austria #367268
Lukaszcyk A, Martin H, Diel P, Fory W, Gatzi K, Kristinson H, Muller B, Muntwyler R, Pachlatko JP, Ciba-Geigy AG (1980) Switzerland, Eur. Pat. #12158
Lin K (1976) Process for making 2-cyano-2-hydroximinoacetamide salts. Patent of the USA #3919284
Butour LJ, Wimmer S, Wimmer F, Castan P (1997) Chemico-Biol Interact 104:165–178
Skopenko VV, Palii GK, Gerasimchuk NN, Makats EF, Domashevskaya OA, Rakovskaya RV (1988) Nitrosothiocarbamylcyanmethanid of potassium or sodium which show antimicrobial activity. Patent of the USSR #1405281
Davidson SH (1978) 2-Cyano-2-hydroximinoacetamides as plant disease control agents. Patent of the USA #3957847
Kuhne A, Hubele A (1978) Method for the cultivation of plants employing α-cyanohydroximino -acetamide derivatives. Patent of the USA #4063921
Gerasimchuk NN, Kuzmann E, Bǘki A, Verteś A, Nagy L, Burger K (1991) Synthesis, IR-, Mössbauer studies of Eu3+ complexes formed with cyanoxime anions. Inorg Chim Acta 188(11):45–50
Gerasimchuk NN, Simonov YuA, Dvorkin AA, Rebrova OA (1993) Synthesis, IR spectra and structure of lead(II) complex with amide-cyanoximate-ion ONC(CN)C(O)NH2-. Russ J Inorg Chem 38(2):247–252
Ponomareva VV, Dalley NK, Xiaolan K, Gerasimchuk NN, Domasevitch KV (1996) Synthesis, spectra and crystal structures of complexes including ambidentate benzoyl- cyanoxime anion C6H5C(O)-C(NO)CN-. J Chem Soc Dalton Trans 2351–2359.
Skopenko VV, Ponomareva VV, Simonov YuA, Domasevitch KV, Dvorkin AA (1994) Coordination compounds of thallium(I) and silver(I) with benzoylcyanoxime-anion. Russ J Inorg Chem 39(8):1332–1339
Mokhir AA, Gerasimchuk NN, Pol’shin EV, Domasevitch KV (1994) Synthesis, IR and Mössbauer study of bisorganotin complexes with cyanoximes. Russ J Inorg Chem 39(2):289–293
Gerasimchuk NN, Dalley NK (2004) Demetallation of Ni(II) tetraazamacrocyclic complex by cyanoxime resulting in the formation of a stereospecific trinuclear compound [Na(H2O)6]+[NaNi2L6]- (L= NC-C(NO)-C(O)NH2-). J Coord Chem 57(16):1431–1445
Skopenko VV, Palii GK, Makats EP, Gerasimchuk NN (1986) Outer-sphere tris-diamine complexes of Iron(II) and their antibmicrobial activity. Dokl Acad Nauk Ukr SSR B(2):45–48.
Palii GK, Skopenko VV, Gerasimchuk NN, Makats EF, Domashevskaya OA, Rakovskaya RV (1988) Bis-(Nitrosothiocarbamylcyan-methanid) copper(II) or nickel(II) which exhibit antimicrobial activity. Patent of the USSR, #1405282
Skopenko VV, Palii GK, Gerasimchuk NN, Domashevskaya OA, Makats EF (1989) Di-(Nitrosothiocarbamylcyanmethanid)-di(pyridine)-copper(II) which shows bacteriostatic activity towards Staphilococcus Aureus, and method of preparation of the complex. Patent of the USSR #1487422
Eddings D, Barnes C, Gerasimchuk N, Durham P, Domasevich K (2004) First bivalent palladium and platinum cyanoximates: synthesis, characterization and biological activity. Inorg Chem 43(13):3894–3909
Eddings D (2003) The synthesis, characterization, spectroscopic and biological activity studies of Pt(II) and Pd(II) cyanoximates. M.S. Thesis (154 pp., 32 tables, 17 figures), Department of Chemistry, Southwest Missouri State University, Springfield, Missouri
Ratcliff J (2007) Further investigations of cytotoxic metallocyanoximates. MS Thesis (14 tables, 60 figures). Department of Chemistry, Missouri State University, Springfield, Missouri
Gerasimchuk N, Maher T, Durham P, Domasevitch KV, Wilking J, Mokhir A (2007) Tin(IV) cyanoximates: synthesis, characterization and in vitro cytotoxicity. Inorg Chem 46(18):7268–7284
Snyder J (2007) Synthesis and investigation of several dibutyltin(IV) cyanoximates. MS Thesis (18 tables, 45 figures). Department of Chemistry, Missouri State University, Missouri
Dannen SD, Cornelison L, Durham P, Morley JE, Shahverdi K, Du J, Zhou H, Sudlow LC, Hunter D, Wood MD, Berezin MY, Gerasimchuk N (2020) New in vitro highly cytotoxic platinum and palladium cyanoximates with minimal side effects in vivo. J Inorg Biochem 208:111082. https://doi.org/10.1016/j.jinorgbio.2020.111082
Gerasimchuk N (2019) Chemistry and applications of cyanoximes and their metal complexes. Dalton Trans 48:7985–8013
Charlier H, Gerasimchuk N (2010) Cyanoxime inhibitors of carbonyl reductase and methods of using said inhibitors in treatments involving anthracyclines. USA patent # 7,727,967 B2
Lehmann C, Renk E, Gadneux A (Geigy, J.R. AG) South African Patent #680079 (1968).
Myatt, L.H. (Ciba-Geigy Corp., USA), USA patent #4260555 (1981)
Wang P, Wang R, Dai J, Wu X, Xu J (1996) Yaoxue Xuebao 31:918–924
Wang P, Xu J, Yin W, Wu X, Wang R (1998) Nongyao 37:14–15
Hackmann TJ, Harthoorn AP, Bay H, Rosinger PH (1966) USA patent #3234255
Myatt LH (1981) USA patent #4260555
Freenor JF, Koerber MB (1978) USA patent #4123255
Goeden L (2005) The synthesis, characterization and bilogical activity studies of Pt(II) and Pd(II) disubstituted arylcyanoximates. M.S. Thesis (195 pp., 27 tables, 64 figures), Department of Chemistry, Southwest Missouri State University, Springfield, Missouri
Gordon AJ, Ford RA (1972) The chemist companion (Handbook). Wiley, New York
Kosower EM, Hoffmann D, Wallenfels K (1961) The effect of solvent on spectra. VII. the “methyl effect” in the spectra of dihydropyridines. J Am Chem Soc 83(15):3314–3319
Gerasimchuk N, Guzei I, Sipos P (2015) Structural peculiarities of cyanoximes and their anions: co-crystallization of two diastereomers and formation of acid-salts. Curr Inorg Chem 5(1):38–63
Robertson D, Barnes C, Gerasimchuk N (2004) Synthesis of the monosubstituted arylcyanoximes and its Na, Tl(I) and Ag(I) compounds. J Coord Chem 57(14):1205–1216
Gerasimchuk N, Goeden L, Durham P, Barnes C, Cannon JF (2008) Synthesis and characterization of the first disubstituted arylcyanoximes and their several metal complexes. Inorg Chim Acta 361:1983–2001
Robertson D, Cannon J, Gerasimchuk N (2005) Double-stranded metal-organic networks for one-dimensional mixed valence coordination polymers. Inorg Chem 44(23):8326–8342
Domashevskaya OA, Mazus MD, Gerasimchuk NN, Dvorkin AA, Simonov YuA (1989) Crystal and molecular structure of Cesium and Rubidium nitrosocarbamoyl-cyan methanides. Russ J Inorg Chem 34(7):1656–1660
Morton J, Dennison R, Nemykin V, Gerasimchuk N (2020) Planochromism of cyanoxime anions: computational and mechanistic studies in solid state and solutions. Inorg Chim Acta. https://doi.org/10.1016/j.ica.2020.119570
Robertson D (2006) Thallium(I) coordination polymers based on monosubstituted arylcyanoximes. M.S. Thesis, Department of Chemistry, Missouri State University, Springfield, Missouri
Reichardt C, Welton T (2010) Solvents and solvent effects in organic chemistry (4th updated edn). Wiley, Weinheim, pp 360. ISBN 9783527324736.
Bakhsiev Solvatochromism: problems and methods. Izd-vo Leningradskogo Universiteta: Leningrad, USSR NG (1989) Chem Abstr 1990(112):186279g
Gerasimchuk N, Esaulenko AN, Dalley KN, Moore C (2010) 2-Cyano-2-isonitroso acetamide and its Ag(I) complexes. Silver(I) cyanoximate as a non-electric gas sensor. Dalton Trans 39:449–764
Mann A, Gerasimchuk N, Silchenko S (2016) New non-aggregating bivalent cis-ML2 (M = Pd, Pt; L = pivaloylcyanoxime). Inorg Chim Acta 440:118–128
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) OLEX2: a complete structure solution, refinement and analysis program. J Appl Cryst 42(2):229–341
Acknowledgements
NG is grateful to Mr. Leon Goeden for his pioneering work on the project, Dr. Svitlana Silchenko (Absorption Systems, Inc.) for pK determination and Ms. Victoria Barry for technical help. A financial support from the ACS PRF Award # 39079-B3 and the Cottrell Research Corporation Award # CC6598 is highly appreciated. NG is also very grateful to the College of Natural and Applied Sciences for the continuous support of the X-ray analysis facility.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Amankrah, S.A., Hietsoi, O., Tyukhtenko, S. et al. Preparation, Properties and Crystal Structure of syn-Isomer of 2,6-Dichlorophenyl-cyanoxime, H(2,6-diCl-PhCO): Potent Carbonyl Reductase Inhibitor. J Chem Crystallogr 52, 194–204 (2022). https://doi.org/10.1007/s10870-021-00905-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-021-00905-1