Abstract
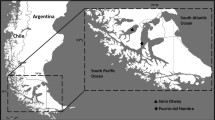
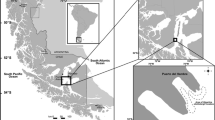
Macrocystis pyrifera (L.) C. Agardh is a species that forms extensive underwater forests along the coastline of the ecoregion of Magellan. There, this alga is exposed to marked variation in photoperiod, temperature, and irradiance, which are modulated by daily and seasonal climatic variations. This study aims to understand the ecophysiological behavior of M. pyrifera, in a natural forest localized in Puerto del Hambre, Magellan Region of Chile, along spatial (depth) and temporal (season) gradients of physical drivers by analyzing algal responses in terms of the photosynthetic pigments and fluorescence yield. In the apical, middle, and basal fronds, the following photosynthetic parameters were seasonally measured: electron transport efficiency (α), maximum relative rate of electron transport (rETRmax), saturation point (E k ), and pigments such as chlorophyll a (Chl a), chlorophyll c (Chl c), and fucoxanthin. Both seasonal and stratified variations were observed. In autumn, α was decreased in the middle fronds (0.136 ± 0.030 (μmol photons m−2 s−1)−1) with respect to apical and basal fronds of autumn. For parameters such as E k , this decrease was observed relative to the depth gradient, with significant differences (p < 0.05) between distinct fronds. rETRmax was high in the apical fronds in spring, autumn, and winter. High Chl a concentration was maintained in all seasons, while the concentration of Chl c in the apical fronds tended to be lower. The concentration of fucoxanthin remained stable without significant differences between dissimilar types of fronds, for seasons (spring, summer, autumn, and winter). The Chl a/Chl c ratio increased with depth, while the Chl a/fucoxanthin ratio varies seasonally. Variations of light intensity in a natural population in a depth gradient and pigment variations in M. pyrifera with the depth stratification reveal the behavior of these algae in the ecoregion of Magellan where Chl a, through the apical fronds, could be regulating the photosynthetic activity of the plants at stratification level. Furthermore, the increase in Chl c and fucoxanthin towards the middle and basal fronds showed similar trends as those measured for α, thus signifying higher photosynthetic efficiency at greater depth. Overall, our results indicate marked seasonal and depth acclimation to different environmental conditions. This study is the first of its kind for the ecoregion of Magellan in which M. pyrifera represents a keystone species of utmost ecological significance.







Similar content being viewed by others
References
Anderson JM, Chow WS, Park YI (1995) The grand design of photosynthesis: acclimation of the photosynthetic apparatus to environmental cues. Photosynth Res 46:129–139
Becker S, Graeve M, Bishof K (2010) Photosynthesis and lipid composition of the Antarctic endemic rhodophyte Palmaria decipiens, effects of changing light, and temperature levels. Polar Biol 33:945–955
Beer S, Björk M, Beardall J (2014) Photosynthesis in the marine environment. Wiley, Oxford, pp 61–64
Bozinovic F, Calosi P, Spicer J (2011) Physiological correlates of geographic range in animals. Annu Rev Ecol Evol Syst 42:155–179
Buschmann A, Pereda S, Varela D, Rodriguez J, López A, González L, Schilling M, Henríquez E, Hernández M (2014) Ecophysiological plasticity of annual populations of giant kelp (Macrocystis pyrifera) in a seasonally variable coastal environment in the Northern Patagonian Inner Seas of Southern Chile. J Appl Phycol 26:837–847
Cabello-Pasini A, Aguirre-von-Wobeser E, Figueroa F (2000) Photoinhibition of photosynthesis in Macrocystis pyrifera (Phaeophyceae), Chondrus crispus (Rhodophyceae) and Ulva lactuca (Chlorophyceae) in outdoor culture systems. J Photochem Photobiol 57:169–178
Castelo D, Trigueiro T, Colepicolo P, Marinho Soriano E (2012) Seasonal changes in the pigment composition of natural population of Gracilaria domingensis (Gracilariales, Rhodophyta). Braz J Pharmacog 22:874–880
Claudet J, Pelletier D, Jouvenel JY, Bachet F, Galzin R (2006) Assessing the effects of marine protected area (mpa) on a reef fish assemblage in a Northwestern Mediterranean marine reserve: identifying community-based indicators. Biol Conserv 130:349–369
Colombo-Pallotta M, García Mendoza E, Ladah L (2006) Photosynthetic performance, light absorption, and pigment composition of Macrocystis pyrifera (Laminariales, Phaeophyceae) blades from different depths. J Phycol 42:1225–1234
Delgado-Vargas F, Jiménez A, Paredes-López O (2000) Natural pigments: carotenoids, anthocyanins, and betalains—characteristics, biosynthesis, processing, and stability. Crit Rev Food Sci Nutr 40:173–289
Edwards M, Kim K (2010) Diurnal variation in relative photosynthetic performance in giant kelp Macrocystis pyrifera (Phaeophyceae, Laminariales) at different depths as estimated using PAM fluorometry. Aquat Bot 92:119–128
Fernandes F, Barbosa M, Oliveira AP, Azevedo IC, Sousa-Pinto I, Valentão P, Andrade PB (2016) The pigments of kelps (Ochrophyta) as part of the flexible response to highly variable marine environments. J Appl Phycol 28:3689–3696
Frank H, Chynwat V, Desamero R, Farhoosh R, Erickson J, Bautista J (1997) On the photophysics and photochemical properties of carotenoids and their role as light–harvesting pigments in photosynthesis. Pure Appl Chem 69:2117–2124
Gao K, Umezaki I (1988) Comparative photosynthetic capacities of the leaves of upper and lower parts of Sargassum plants. Bot Mar 31:231–236
Gerard V (1984) The light environment in a giant kelp forest: influence of Macrocystis pyrifera on spatial and temporal variability. Mar Biol 84:189–195
Graham M, Vasquez J, Buschmann A (2007) Global ecology of the giant kelp Macrocystis: from ecotypes to ecosystems. Oceanogr Mar Biol 45:39–88
Griffiths M, Harrison S, Smit M, Maharajh D (2016) Major commercial products from micro–and macroalgae. In: Bux F, Chisti Y (eds) Algae biotechnology. Springer, Cham, pp 269–300
Guimarães SMPB (2003) Uma análise da diversidade da flora marinha bentônica do estado do Espírito Santo, Brasil. Hoehnea 30:11–19
Gunnarsson K, Ingólfsonn A (1995) Seasonal changes in the abundance of intertidal algae in Southwestern Iceland. Bot Mar 38:69–77
Gupta S, Abu-Ghannam N (2011) Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci Technol 22:315–326
Karsten U (2007) Research note: salinity tolerance of Arctic kelps from Spitsbergen. Phycol Res 55:257–262
Karsten U (2012) Seaweed acclimation to salinity and desiccation stress. In: Wiencke C, Bishoff K (eds) Seaweed biology. Springer, Berlin, pp 87–107
Kim S, Bhatnagar I (2011) Physical, chemical, and biological properties of wonder kelp—Laminaria. Adv Food Nutr Res 64:85
Koch K, Thiel M, Hagen W, Graeve M, Gómez I, Jofre D, Hoffman L, Tala F, Bischof K (2016) Short and long term acclimation patterns of the giant kelp Macrocystis pyrifera (Laminariales, Phaeophyceae) along a depth gradient. J Phycol 52:260–273
Lichtenthaler HK, Babani F (2004) Light adaptation and senescence of the photosynthetic apparatus. Changes in pigment composition, chlorophyll fluorescence parameters and photosynthetic activity. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis. Springer, Dordrecht, pp 713–736
Macaya EC, Zucarello GC (2010) Genetic structure of the giant kelp Macrocystis pyrifera along the southeastern Pacific. Mar Ecol Prog Ser 420:103–112
Manley S (1984) Micronutrient uptake and translocation by Macrocystis pyrifera. J Phycol 20:192–201
Mansilla A, Palacios M, Aguilar S (2004) Efecto de la Salinidad en el Desarrollo Inicial de Sarcothalia Crispata (Bory) Leister (Rhodophyta, Gigartinales) Bajo Condiciones de Laboratorio. An Inst Patagonia 32:13–23
Meléndez-Martínez A, Vicario I, Heredia F (2007) Pigmentos carotenoides: consideraciones estructurales y fisicoquímicas. Arch Latinoam Nutr 57:109–117
Ojeda J (2013) Dinámica estacional de macroalgas y moluscos intermareales y su relación con el conocimiento tradicional ecológico yagán, en canales subantárticos del Cabo de Hornos: una aproximación biocultural desde la filosofía ambiental de campo. Postgraduate Thesis, Universidad de Magellan, Punta Arenas, Chile, 145 pp
Ojeda J, Rosenfeld S, Marambio J, Rozzi R, Mansilla A (2014) Patrones estacionales y espaciales de la diversidad de moluscos intermareales de Bahía Róbalo, canal Beagle, Reserva de la Biosfera Cabo de Hornos, Chile. Rev Biol Mar Ocean 49(3):493–509
Pangestuti R, Kim S (2011) Biological activities and health benefit effects of natural pigments derived from marine algae. J Funct Foods 3:255–266
Platt T, Gallegos CL, Harrison WG (1980) Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J Mar Res 38:687–701
Quitral V, Morales C, Sepúlveda M, Schwartz M (2012) Propiedades nutritivas y saludables de algas marinas y su potencialidad como ingrediente funcional. Rev Chil Nutr 39:196–202
Ralph PJ, Gademann R (2005) Rapid light curves: a powerful tool to assess photosynthetic activity. Aquat Bot 82:222–237
Ramlov F, de Souza J, Farias A, Maraschin M, Horta P, Yokoya N (2012) Effects of temperature, salinity, irradiance, and nutrients on the development of carposporelings and tetrasporophytes in Gracilaria domingensis (Kütz.) Sonder ex Dickie (Rhodophyta, Gracilariales). Bot Mar 55:253–259
Ramus J, Lemons F, Zimmerman C (1977) Adaptation of light-harvesting pigments to downwelling light and the consequent photosynthetic performance of the eulittoral rockweeds Ascophyllum nodosum and Fucus vesiculosus. Mar Biol 42:293–293
Rautenberger R, Bischof K (2016) Dynamic summer solar radiation in Antarctic coastal ecosystems and its effects on photosynthesis of the endemic Antarctic brown macroalga Desmarestia menziesii (Phaeophyceae). Algol Stud 151-152:123–150
Rautenberger R, Mansilla A, Gómez I, Wiencke C, Bischof K (2009) Photosynthetic responses to UV- radiation of intertidal macroalgae from the Strait of Magellan (Chile). Rev Chil Hist Nat 82:43–61
Reiskind JB, Madsen TV, Van Ginkel LC, Bowes G (1997) Evidence that inducible C4-type photosynthesis is a chloroplastic CO2- concentrating mechanism in Hydrilla, a submersed monocot. Plant Cell Environ 20:211–220
Rothäusler E, Gómez I, Hinojosa I, Karsten U, Tala F, Thiel M (2011a) Physiological performance of floating giant kelp Macrocystis pyrifera (Phaeophyceae): latitudinal variability in the effects of temperature and grazing1. J Phycol 47:269–281
Rothäusler E, Gómez I, Karsten U, Tala F, Thiel M (2011b) Physiological acclimation of floating Macrocystis pyrifera to temperature and irradiance ensures long–term persistence at the sea surface at mid–latitudes. J Exp Mar Biol Ecol 405:33–41
Russell G (1986) Variation and natural selection in marine macroalgae. Oceanogr Mar Biol Annu Rev 24:309–377
Seely GR, Duncan MJ, Vidaver WE (1972) Preparative and analytical extraction of pigments from brown algae with dimethyl sulfoxide. Mar Biol 12:184–188
Sexton J, McIntyre P, Angert RK (2009) Evolution and ecology of species range limits. Annu Rev Ecol Evol Syst 40:415–436
Skene K (2004) Key differences in photosynthetic characteristics of nine species of intertidal macroalgae are related to their position on the shore. Can J Bot 82:177–184
Vergara J (2003) Tipología y clasificación de fiordos y piedmonts submarinos de Magellan. Chile Invest Geogr Chile 37:21
Wheeler WN (1980) Pigment content and photosynthetic of the frond of Macrocystis pyrifera. Mar Biol 56:97–102
Yokoya NS, Necchi O Jr, Martins AP, Gonzalez SF, Plastino EM (2007) Growth responses and photosynthetic characteristics of wild and phycoerythrin-deficient strains of Hypnea musciformis (Rhodophyta). J Appl Phycol 19:197–205
Acknowledgements
The authors wish to thank to the project FONDECYT 1140940 “Macroalgal adaptive radiation: potential links to ecological niche diversity in the ecoregion of Magallanes and Chilean Antarctic.” We are thankful for the graduate scholarships by the Institute of Ecology and Biodiversity granted to FM (ICM, P05-002) and JPR (ICM, P05-002) and to JM (PFB-23-2008) and SR (ICM P05-002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marambio, J., Rodriguez, J.P., Mendez, F. et al. Photosynthetic performance and pigment composition of Macrocystis pyrifera (Laminariales, Phaeophyceae) along a gradient of depth and seasonality in the ecoregion of Magellan, Chile. J Appl Phycol 29, 2575–2585 (2017). https://doi.org/10.1007/s10811-017-1136-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1136-0