Abstract
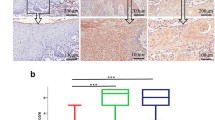
The present study aimed to detect the expression of interleukin-27 (IL-27) in tissues of oral lichen planus (OLP), oral leukoplakia (OLK), and oral squamous cell carcinoma (OSCC) and to investigate the possible role of IL-27 in the above diseases and whether it was involved in the onset and development of the tumor. Paraffin tissues from patients with OLP, OLK, and OSCC were collected, and the expression of IL-27 in the above tissues was detected by immunohistochemical (IHC) staining. Parameters were obtained from the images by the Image-Pro Plus (IPP) image analysis software, and statistical analysis was performed using relevant data. The expressions of IL-27 were significantly higher in specimens with OLP, OLK, and OSCC than those in the healthy group. In OLP, the expression of IL-27 was positively correlated with the degree of lymphocyte infiltration and basal cell liquefaction while independent of the clinical type. In OLK, the expression of IL-27 was positively correlated with abnormal epithelial cell proliferation. In OSCC, the expression of IL-27 was correlated with the degree of squamous cell differentiation and was independent of gender, TNM stage, and lymphatic metastasis. The expressions of IL-27 were significantly higher in tissues with severe OLP and OLK than that in the control group, while similar to that in highly differentiated OSCC. The expressions of IL-27 were significantly elevated in tissues with OLP, OLK, and OSCC, suggesting that IL-27 might be involved in the development of these diseases and play a role in the carcinogenesis of oral precancerous lesions.









Similar content being viewed by others
References
Fabbi, M., G. Carbotti, and S. Ferrini. 2017. Dual roles of IL-27 in cancer biology and immunotherapy. Mediators of Inflammation 2017: 3958069.
Lai, X., H. Wang, J. Cao, et al. 2016. Circulating IL-27 is elevated in rheumatoid arthritis patients. Molecules 21 (11): 1565.
Andrews, C., M.H. McLean, and S.K. Durum. 2016. Interleukin-27 as a novel therapy for inflammatory bowel disease: A critical review of the literature. Inflammatory Bowel Diseases 22 (9): 2255–2264.
Lu, D., X. Zhou, L. Yao, C. Liu, F. Jin, and Y. Wu. 2014. Clinical implications of the interleukin 27 serum level in breast cancer. Journal of Investigative Medicine 62 (3): 627–631.
Garg, A., R. Trout, and S.A. Spector. 2017. Human immunodeficiency virus type-1 myeloid derived suppressor cells inhibit cytomegalovirus inflammation through interleukin-27 and B7–H4. Science and Reports 7: 44485.
Huang, Z., J. Zak, I. Pratumchai, et al. 2019. IL-27 promotes the expansion of self-renewing CD8+ T cells in persistent viral infection. Journal of Experimental Medicine 216 (8): 1791–1808.
Li, M.S., Z. Liu, J.Q. Liu, X. Zhu, Z. Liu, and X.F. Bai. 2015. The Yin and Yang aspects of IL-27 in induction of cancer-specific T-cell responses and immunotherapy. Immunotherapy 7 (2): 191–200.
Han, Y., T. Su, L. Lin, et al. 2016. Clinical study on cervical lymph nodes metastasis of oral squamous cell carcinoma. Journal of Oral Science Research 32(02):154–157 (Article in China). https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2016&filename=KQYZ201602016&v=ixgP%25mmd2Fpg844H6u%25mmd2FrQ%25mmd2Bur0zDT9df0D%25mmd2Foi2vHDXnhAokQSgdOhnGIxtGEaXSNyuYwTj
Jemal, A., F. Bray, M.M. Center, J. Ferlay, E. Ward, D. Forman. 2011. Global cancer statistics [published correction appears in CA: a Cancer Journal for Clinicians 2011 61 (2): 134]. CA: a Cancer Journal for Clinicians 61 (2): 69–90.
Cheng, Y.S., A. Gould, Z. Kurago, J. Fantasia, and S. Muller. 2016. Diagnosis of oral lichen planus: A position paper of the American Academy of Oral and Maxillofacial Pathology. Oral Surgery, Oral Medicine, Oral Pathology, and Oral Radiology 122 (3): 332–354.
González-Moles, M.Á., P. Ramos-García, and S. Warnakulasuriya. 2020. An appraisal of highest quality studies reporting malignant transformation of oral lichen planus based on a systematic review. Oral Diseases. https://doi.org/10.1111/odi.13741.
Dong, Y., Y. Chen, Y. Tao, et al. 2019. Malignant transformation of oral leukoplakia treated with carbon dioxide laser: A meta-analysis. Lasers in Medical Science 34 (1): 209–221.
Sharma, R., P. Narang, A.K. Sharma, et al. 2014. Prevalence of leukoplakia in patients visiting dental college in rural area of Jaipur, Rajasthan. Indian Journal of Public Health Research & Development. 5 (3): 292–295.
Burgdorf, W.H., and G. Plewig. 2014. Who described civatte bodies? Journal of Cutaneous Pathology 41 (4): 340–346.
van der Waal, I. 2015. Oral leukoplakia, the ongoing discussion on definition and terminology. Medicina Oral, Patología Oral y Cirugía Bucal 20 (6): e685–e692.
Tang, H., J. Mao, L. Gao, J. Liu, and T. Wu. 2013. Effect of antisense TIMP-1 cDNA on the expression of TIMP-1 and MMP-2 in lung tissue with pulmonary fibrosis induced by bleomycin. Molecular Medicine Reports 7 (1): 149–153.
Schmidt-Westhausen, A.M. 2020. Oral lichen planus and lichenoid lesions: What’s new? Quintessence International 51 (2): 156–161.
Piccinni, M.P., L. Lombardelli, F. Logiodice, et al. 2014. Potential pathogenetic role of Th17, Th0, and Th2 cells in erosive and reticular oral lichen planus. Oral Diseases 20 (2): 212–218.
Mashhadiabbas, F., and M. Fayazi-Boroujeni. 2017. Correlation of vascularization and inflammation with severity of oral leukoplakia. Iranian Journal of Pathology 12 (3): 225–230.
Coppock, G.M., L.R. Aronson, J. Park, J.H. DeLong, E. Radaelli, K. Suszták, C.A. Hunter, et al. 2020. Loss of IL-27Rα results in enhanced tubulointerstitial fibrosis associated with elevated Th17 responses. The Journal of Immunology 205 (2): 377–386.
Santana, P.T., J. Martel, H.C. Lai, J.L. Perfettini, J.M. Kanellopoulos, J.D. Young, R. Coutinho-Silva, and D.M. Ojcius. 2016. Is the inflammasome relevant for epithelial cell function? Microbes and Infection 18 (2): 93–101.
Zhang, B., F. Xie, C.L. Dong, et al. 2017. The cross-talk between cervical carcinoma cells and vascular endothelial cells mediated by IL-27 restrains angiogenesis. American Journal of Reproductive Immunology 78 (4).https://doi.org/10.1111/aji.12706.
Xia, H., Z.J. Ye, Q. Zhou, et al. 2014. IL-27 and IL-27-producing CD4+ T cells in human tuberculous pleural effusion. Tuberculosis (Edinburgh, Scotland) 94 (6): 579–588.
de Aquino, M.T., P. Kapil, D.R. Hinton, et al. 2014. IL-27 limits central nervous system viral clearance by promoting IL-10 and enhances demyelination. The Journal of Immunology 193 (1): 285–294.
Nguyen, Q.T., E. Jang, H.T. Le, et al. 2019. IL-27 targets Foxp3+ Tregs to mediate anti-inflammatory functions during experimental allergic airway inflammation [published online ahead of print, 2019 Jan 24]. JCI Insight 4 (2): e123216.
Pekiner, F.N., G.Y. Demirel, M.O. Borahan, and S. Ozbayrak. 2012. Cytokine profiles in serum of patients with oral lichen planus. Cytokine 60 (3): 701–706.
Tao, X.A., J. Xia, X.B. Chen, et al. 2010. FOXP3 T regulatory cells in lesions of oral lichen planus correlated with disease activity. Oral Diseases 16 (1): 76–82.
Sun, Y., N. Liu, X. Guan, H. Wu, Z. Sun, and H. Zeng. 2016. Immunosuppression induced by chronic inflammation and the progression to oral squamous cell carcinoma. Mediators of Inflammation 2016: 5715719.
Cui, B., S. Lu, L. Lai, et al. 2017. Protective function of interleukin 27 in colitis-associated cancer via suppression of inflammatory cytokines in intestinal epithelial cells. Oncoimmunology. 6 (2): e1268309.
Wang, T., Y. Chen, H. Nie, Y. Huang, Y. Zhao, and J. Yang. 2019. IL-27 inhibits non-small-cell lung cancer cell metastasis by miR-935 in vitro. Oncotargets and Therapy 12: 1447–1454.
Ge, H., N. Yin, T.L. Han, et al. 2019. Interleukin-27 inhibits trophoblast cell invasion and migration by affecting the epithelial-mesenchymal transition in preeclampsia. Reproductive Sciences 26 (7): 928–938.
Di Carlo, E., C. Sorrentino, A. Zorzoli, et al. 2014. The antitumor potential of Interleukin-27 in prostate cancer. Oncotarget 5 (21): 10332–10341.
Goertzen, C., H. Mahdi, C. Laliberte, et al. 2018. Oral inflammation promotes oral squamous cell carcinoma invasion. Oncotarget 9 (49): 29047–29063.
Qian, Y., W. Yao, T. Yang, et al. 2017. aPKC-ι/P-Sp1/snail signaling induces epithelial-mesenchymal transition and immunosuppression in cholangiocarcinoma. Hepatology 66 (4): 1165–1182.
Park, Y.J., H. Ryu, G. Choi, et al. 2019. IL-27 confers a protumorigenic activity of regulatory T cells via CD39. Proceeding of the National Academy of Sciences U S A. 116 (8): 3106–3111.
Babadi, A.S., A. Kiani, E. Mortaz, et al. 2019. Serum interleukin-27 level in different clinical stages of lung cancer. Open Access Macedonian Journal of Medical Sciences 7 (1): 45–49.
Kopiński, P., T. Wandtke, A. Dyczek, et al. 2018. Increased levels of interleukin 27 in patients with early clinical stages of non-small cell lung cancer. Polish Archives of Internal Medicine 128 (2): 105–114.
Bisevac, J.P., I. Stanojevic, Z. Mijuskovic, T. Banovic, M. Djukic, and D. Vojvodic. 2016. High interleukin 27 production is associated with early clinical stage and localized disease in patients with melanoma. Journal of Medical Biochemistry 35 (4): 443–450.
Acknowledgements
We are particularly grateful to all the people who have given us help with our article.
Funding
This work was supported by the Fujian Foundation for Medical Innovation (No: 2018-CXB-7).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The study was conducted in accordance with the Declaration of Helsinki (as was revised in 2013). The study was approved by the Ethics Committee of the Union Hospital, Fujian Medical University, and informed consent was taken from all the patients.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10753_2021_1599_MOESM1_ESM.jpg
Supplementary file1 (JPG 9432 KB) Negative control (isotype control) figure of immunohistochemistry. All groups were stained non-specifically using an isotype control. a,b: OLP reticular type c,d: OLP atrophic erosive type e,f: OLK-S group; g,h: OLK-D group; i,j: OSCC-W group; k,l: OSCC-P group.
Rights and permissions
About this article
Cite this article
Wang, QM., Huang, XY. & Guan, WQ. Expressions of Interleukin-27 in Oral Lichen Planus, Oral Leukoplakia, and Oral Squamous Cell Carcinoma. Inflammation 45, 1023–1038 (2022). https://doi.org/10.1007/s10753-021-01599-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01599-5